.

Worlds Within Worlds:
The Story of Nuclear Energy
Volume 1
Atomic Weights · Energy · Electricity
by Isaac Asimov
U. S. Energy Research and Development Administration
Office of Public Affairs
Washington, D.C. 20545
Library of Congress Catalog Card Number: 75-189477
1972
Nothing in the history of mankind has opened our eyes to the possibilities of science as has the development of atomic power. In the last 200 years, people have seen the coming of the steam engine, the steamboat, the railroad locomotive, the automobile, the airplane, radio, motion pictures, television, the machine age in general. Yet none of it seemed quite so fantastic, quite so unbelievable, as what man has done since 1939 with the atom ... there seem to be almost no limits to what may lie ahead: inexhaustible energy, new worlds, ever-widening knowledge of the physical universe. Isaac Asimov

The U. S. Energy Research and Development Administration publishes a series of booklets for the general public.
Please write to the following address for a title list or for information on a specific subject:
USERDA—Technical Information Center
P. O. Box 62
Oak Ridge, Tennessee 37830

ISAAC ASIMOV received his academic degrees from Columbia University and is Associate Professor of Biochemistry at the Boston University School of Medicine. He is a prolific author who has written over 150 books in the past 20 years, including about 20 science fiction works, and books for children. His many excellent science books for the public cover subjects in mathematics, physics, astronomy, chemistry, and biology, such as The Genetic Code, Inside the Atom, Building Blocks of the Universe, Understanding Physics, The New Intelligent Man’s Guide to Science, and Asimov’s Biographical Encyclopedia of Science and Technology.
In 1965 Dr. Asimov received the James T. Grady Award of the American Chemical Society for his major contribution in reporting science progress to the public.

- VOLUME 1
- Introduction 5
- Atomic Weights 6
- Electricity 11
- Units of Electricity 11
- Cathode Rays 13
- Radioactivity 17
- The Structure of the Atom 25
- Atomic Numbers 30
- Isotopes 35
- Energy 47
- The Law of Conservation of Energy 47
- Chemical Energy 50
- Electrons and Energy 54
- The Energy of the Sun 55
- The Energy of Radioactivity 57
- VOLUME 2
- Mass and Energy 69
- The Structure of the Nucleus 75
- The Proton 75
- The Proton-Electron Theory 76
- Protons in Nuclei 80
- Nuclear Bombardment 82
- Particle Accelerators 86
- The Neutron 92
- Nuclear Spin 92
- Discovery of the Neutron 95
- The Proton-Neutron Theory 98
- The Nuclear Interaction 101
- Neutron Bombardment 107
- VOLUME 3
- Nuclear Fission 117
- New Elements 117
- The Discovery of Fission 122
- The Nuclear Chain Reaction 127
- The Nuclear Bomb 131
- Nuclear Reactors 141
- Nuclear Fusion 147
- The Energy of the Sun 147
- Thermonuclear Bombs 149
- Controlled Fusion 151
- Beyond Fusion 159
- Antimatter 159
- The Unknown 164
- Reading List 166

A total eclipse of the sun.
INTRODUCTION
In a way, nuclear energy has been serving man as long as he has existed. It has served all of life; it has flooded the earth for billions of years. The sun, you see, is a vast nuclear engine, and the warmth and light that the sun radiates is the product of nuclear energy.
In order for man to learn to produce and control nuclear energy himself, however (something that did not take place until this century), three lines of investigation—atoms, electricity, and energy—had to develop and meet.
We will begin with atoms.
ATOMIC WEIGHTS
As long ago as ancient Greek times, there were men who suspected that all matter consisted of tiny particles which were far too small to see. Under ordinary circumstances, they could not be divided into anything smaller, and they were called “atoms” from a Greek word meaning “indivisible”.
It was not until 1808, however, that this “atomic theory” was really put on a firm foundation. In that year the English chemist John Dalton (1766-1844) published a book in which he discussed atoms in detail. Every element, he suggested, was made up of its own type of atoms. The atoms of one element were different from the atoms of every other element. The chief difference between the various atoms lay in their mass, or weight.[1]
Dalton was the first to try to determine what these masses might be. He could not work out the actual masses in ounces or grams, for atoms were far too tiny to weigh with any of his instruments. He could, however, determine their relative weights; that is, how much more massive one kind of atom might be than another.
For instance, he found that a quantity of hydrogen gas invariably combined with eight times its own mass of oxygen gas to form water. He guessed that water consisted of combinations of 1 atom of hydrogen with 1 atom of oxygen. (A combination of atoms is called a “molecule” from a Greek word meaning “a small mass”, and so hydrogen and oxygen atoms can be said to combine to form a “water molecule”.)

John Dalton
To account for the difference in the masses of the combining gases, Dalton decided that the oxygen atom was eight times as massive as the hydrogen atom. If he set the mass of the hydrogen atom at 1 (just for convenience) then the mass of the oxygen atom ought to be set at 8. These comparative, or relative, numbers were said to be “atomic weights”, so that what Dalton was suggesting was that the atomic weight of hydrogen was 1 and the atomic weight of oxygen was 8. By noting the quantity of other elements that combined with a fixed mass of oxygen or of hydrogen, Dalton could work out the atomic weights of these elements as well.
Dalton’s idea was right, but his details were wrong in some cases. For instance, on closer examination it turned out that the water molecule was composed of 1 oxygen atom and 2 hydrogen atoms. For this reason, the water molecule may be written H₂O, where H is the chemical symbol for a hydrogen atom, and O for an oxygen atom.
It is still a fact that a quantity of hydrogen combines with eight times its mass of oxygen, so the single oxygen atom must be eight times as massive as the 2 hydrogen atoms taken together. The oxygen atom must therefore be sixteen times as massive as a single hydrogen atom. If the atomic weight of hydrogen is 1, then the atomic weight of oxygen is 16.
At first it seemed that the atomic weights of the various elements were whole numbers and that hydrogen was the lightest one. It made particular sense, then, to consider the atomic weight of hydrogen as 1, because that made all the other atomic weights as small as possible and therefore easy to handle.
The Swedish chemist Jöns Jakob Berzelius (1779-1848) continued Dalton’s work and found that elements did not combine in quite such simple ratios. A given quantity of hydrogen actually combined with a little bit less than eight times its mass of oxygen. Therefore if the atomic weight of hydrogen were considered to be 1, the atomic weight of oxygen would have to be not 16, but 15.87.

Jöns Jakob Berzelius
As it happens, oxygen combines with more elements (and more easily) than hydrogen does. The ratio of its atomic weight to that of other elements is also more often a whole number. In working out the atomic weight of elements it was therefore more convenient to set the atomic weight of oxygen at a whole number than that of hydrogen. Berzelius did this, for instance, in the table of atomic weights he published in 1828. At first he called the atomic weight of oxygen 100. Then he decided to make the atomic weights as small as possible, without allowing any atomic weight to be less than 1. For that reason, he set the atomic weight of oxygen at exactly 16 and in that case, the atomic weight of 10 hydrogen had to be placed just a trifle higher than 1. The atomic weight of hydrogen became 1.008. This system was retained for nearly a century and a half.
Throughout the 19th century, chemists kept on working out atomic weights more and more carefully. By the start of the 20th century, most elements had their atomic weights worked out to two decimal places, sometimes three.
A number of elements had atomic weights that were nearly whole numbers on the “oxygen = 16” standard. The atomic weight of aluminum was just about 27, that of calcium almost 40, that of carbon almost 12, that of gold almost 197, and so on.
On the other hand, some elements had atomic weights that were far removed from whole numbers. The atomic weight of chlorine was close to 35.5, that of copper to 63.5, that of iron to 55.8, that of silver to 107.9, and so on.
Throughout the 19th century, chemists did not know why so many atomic weights were whole numbers, while others weren’t. They simply made their measurements and recorded what they found. For an explanation, they had to wait for a line of investigation into electricity to come to fruition.
ELECTRICITY
Units of Electricity
Through the 18th century, scientists had been fascinated by the properties of electricity. Electricity seemed, at the time, to be a very fine fluid that could extend through ordinary matter without taking up any room.
Electricity did more than radiate through matter, however. It also produced important changes in matter. In the first years of the 19th century, it was found that a current of electricity could cause different atoms or different groups of atoms to move in opposite directions through a liquid in which they were dissolved.
The English scientist Michael Faraday (1791-1867) noted in 1832 that a given quantity of electricity seemed to liberate the same number of atoms of a variety of different elements. In some cases, though, it liberated just half the expected number of atoms; or even, in a few cases, just a third.
Scientists began to speculate that electricity, like matter, might consist of tiny units. When electricity broke up a molecule, perhaps a unit of electricity attached itself to each atom. In that case, the same quantity of electricity, containing the same number of units, would liberate the same number of atoms.
In the case of some elements, each atom could attach 2 units of electricity to itself, or perhaps even 3. When that happened a given quantity of electricity would liberate only one-half, or only one-third, the usual number of atoms. (Thus, 18 units of electricity would liberate 18 atoms if distributed 1 to an atom; only 9 atoms if distributed 2 to an atom; and only 6 atoms if distributed 3 to an atom.)
It was understood at the time that electricity existed in two varieties, which were called positive and negative. It appeared that if an atom attached a positive unit of electricity to itself it would be pulled in one direction through the solution by the voltage. If it attached a negative unit of electricity to itself it would be pulled in the other direction.

Michael Faraday
The units of electricity were a great deal more difficult to study than the atomic units of matter, and throughout the 19th century they remained elusive. In 1891, though, the Irish physicist George Johnstone Stoney (1826-1911) suggested that the supposed unit of electricity be given a name at least. He called the unit an “electron”.
Cathode Rays
An electric current flows through a closed circuit of some conducting material, such as metal wires. It starts at one pole of a battery, or of some other electricity generating device, and ends at the other. The two poles are the positive pole or “anode” and the negative pole or “cathode”.
If there is a break in the circuit, the current will usually not flow at all. If, however, the break is not a large one, and the current is under a high driving force (which is called the “voltage”), then the current may leap across the break. If two ends of a wire, making up part of a broken circuit, are brought close to each other with nothing but air between, a spark may leap across the narrowing gap before they actually meet and, while it persists, the current will flow despite the break.
The light of the spark, and the crackling sound it makes, are the results of the electric current interacting with molecules of air and heating them. Neither the light nor the sound is the electricity itself. In order to detect the electricity, the current ought to be forced across a gap containing nothing, not even air.
In order to do that, wires would have to be sealed into a glass tube from which all (or almost all) the air was withdrawn. This was not easy to do and it was not until 1854 that Heinrich Geissler (1814-1879), a German glass-blower and inventor, accomplished this feat. The wires sealed 14 into such a “Geissler tube” could be attached to the poles of an electric generator, and if enough voltage was built up, the current would leap across the vacuum.
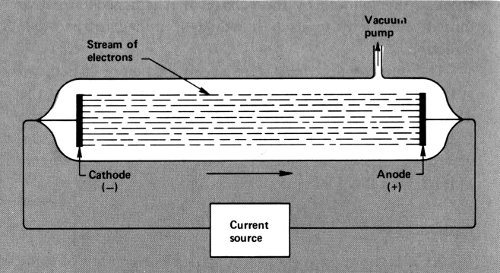
A Geissler tube.
Such experiments were first performed by the German physicist Julius Plücker (1801-1868). In 1858 he noticed that when the current flowed across the vacuum there was a greenish glow about the wire that was attached to the cathode of the generator. Others studied this glow and finally the German physicist Eugen Goldstein (1850-1931) decided in 1876 that there were rays of some sort beginning at the wire attached to the negatively charged cathode and ending at the part of the tube opposite the cathode. He called them “cathode rays”.
These cathode rays, it seemed, might well be the electric current itself, freed from the metal wires that usually carried it. If so, determining the nature of the cathode rays might reveal a great deal about the nature of the electric current. Were cathode rays something like light and were they made up of tiny waves? Or were they a stream of particles possessing mass?
There were physicists on each side of the question. By 1885, however, the English physicist William Crookes 15 (1832-1919) showed that cathode rays could be made to turn a small wheel when they struck that wheel on one side. This seemed to show that the cathode rays possessed mass and were a stream of atom-like particles, rather than a beam of mass-less light. Furthermore, Crookes showed that the cathode rays could be pushed sideways in the presence of a magnet. (This effect, when current flows in a wire, is what makes a motor work.) This meant that, unlike either light or ordinary atoms, the cathode rays carried an electric charge.

J. J. Thomson in his laboratory. On his right are early X-ray pictures.
This view of the cathode rays as consisting of a stream of electrically charged particles was confirmed by another English physicist, Joseph John Thomson (1856-1940). In 1897 he showed that the cathode rays could also be made to take a curved path in the presence of electrically charged 16 objects. The particles making up the cathode rays were charged with negative electricity, judging from the direction in which they were made to curve by electrically charged objects.
Thomson had no hesitation in maintaining that these particles carried the units of electricity that Faraday’s work had hinted at. Eventually, Stoney’s name for the units of electricity was applied to the particles that carried those units. The cathode rays, in other words, were considered to be made up of streams of electrons and Thomson is usually given credit for having discovered the electron.
The extent to which cathode rays curved in the presence of a magnet or electrically charged objects depended on the size of the electric charge on the electrons and on the mass of the electrons. Ordinary atoms could be made to carry an electric charge and by comparing their behavior with those of electrons, some of the properties of electrons could be determined.
There were, for instance, good reasons to suppose that the electron carried a charge of the same size as one that a hydrogen atom could be made to carry. The electrons, however, were much easier to pull out of their straight-line path than the charged hydrogen atom was. The conclusion drawn from this was that the electron had much less mass than the hydrogen atom.
Thomson was able to show, indeed, that the electron was much lighter than the hydrogen atom, which was the lightest of all the atoms. Nowadays we know the relationship quite exactly. We know that it would take 1837.11 electrons to possess the mass of a single hydrogen atom. The electron is therefore a “subatomic particle”; the first of this sort to be discovered.
In 1897, then, two types of mass-containing particles were known. There were the atoms, which made up ordinary matter, and the electrons, which made up electric current.
Radioactivity
Was there a connection between these two sets of particles—atoms and electrons? In 1897, when the electron was discovered, a line of research that was to tie the two kinds of particles together had already begun.
In 1895 the German physicist Wilhelm Konrad Roentgen (1845-1923) was working with cathode rays. He found that if he made the cathode rays strike the glass at the other end of the tube, a kind of radiation was produced. This radiation was capable of penetrating glass and other matter. Roentgen had no idea as to the nature of the radiation, and so called it “X rays”. This name, containing “X” for “unknown”, was retained even after physicists worked out the nature of X rays and found them to be light-like radiation made up of waves much shorter than those of ordinary light.

Antoine Henri Becquerel.
At once, physicists became fascinated with X rays and began searching for them everywhere. One of those involved in the search was the French physicist Antoine Henri Becquerel (1852-1908). A certain compound, potassium uranyl sulfate, glowed after being exposed to sunlight and Becquerel wondered if this glow, like the glow on the glass in Roentgen’s X-ray tube, contained X rays.

Wilhelm Roentgen and his laboratory at the University of Würzburg.
It did, but while investigating the problem in 1896, Becquerel found that the compound was giving off invisible penetrating X-ray-like radiation continually, whether it was exposed to sunlight or not. The radiation was detected because it would fog a photographic plate just as light would. What’s more, the radiation would fog the plate, even if the plate were wrapped in black paper, so that it could penetrate matter just as X rays could.
Others, in addition to Becquerel, were soon investigating the new phenomenon. In 1898 the Polish (later French) 20 physicist Marie Sklodowska Curie (1867-1934) showed that it was the uranium atom that was the source of the radiation, and that any compound containing the uranium atom would give off these penetrating rays.
Until then, uranium had not been of much interest to chemists. It was a comparatively rare metal that was first discovered in 1789 by the German chemist Martin Heinrich Klaproth (1743-1817). It had no particular uses and remained an obscure element. As chemists learned to work out the atomic weights of the various elements, they found, however, that, of the elements then known, uranium had the highest atomic weight of all—238.
Once uranium was discovered to be an endless source of radiation, it gained interest that has risen ever since. Madame Curie gave the name “radioactivity” to this phenomenon of continuously giving off rays. Uranium was the first element found to be radioactive.
It did not remain alone, however. It was soon shown that thorium was also radioactive. Thorium, which had been discovered in 1829 by Berzelius, was made up of atoms that were the second most massive known at the time. Thorium’s atomic weight is 232.
But what was the mysterious radiation emitted by uranium and thorium?
Almost at once it was learned that whatever the radiation was, it was not uniform in properties. In 1899 Becquerel (and others) showed that, in the presence of a magnet, some of the radiation swerved in a particular direction. Later it was found that a portion of it swerved in the opposite direction. Still another part didn’t swerve at all but moved on in a straight line.
The conclusion was that uranium and thorium gave off three kinds of radiation. One carried a positive charge of electricity, one a negative charge, and one no charge at all. The New Zealand-born physicist Ernest Rutherford (1871-1937) called the first two kinds of radiation “alpha rays” and “beta rays”, after the first two letters of the Greek alphabet. The third was soon called “gamma rays” after the third letter.

Ernest Rutherford

Marie Curie and her two daughters, Eve (left) and Irene, in 1908.

Pierre Curie during a class lecture in 1906, the year of his death.
The gamma rays eventually turned out to be another light-like form of radiation, with waves even shorter than those of X rays. The alpha rays and beta rays, which carried electric charges, seemed to be streams of charged particles (“alpha particles” and “beta particles”) just as the cathode rays had turned out to be.
In 1900, indeed, Becquerel studied the beta particles and found them to be identical in mass and charge with electrons. They were electrons.
By 1906 Rutherford had worked out the nature of the alpha particles. They carried a positive electric charge that was twice as great as the electron’s negative charge. If an electron carried a charge that could be symbolized as -, then the charge of the alpha particle was ++. Furthermore, the alpha particle was much more massive than the electron. It was, indeed, as massive as a helium atom (the second lightest known atom) and four times as massive as a hydrogen atom. Nevertheless, the alpha particle can penetrate matter in a way in which atoms cannot, so that it seems much smaller in diameter than atoms are. The alpha particle, despite its mass, is another subatomic particle.
Here, then, is the meeting point of electrons and of atoms—the particles of electricity and of matter.
Ever since Dalton had first advanced the atomic theory over a century earlier, chemists had assumed that atoms were the fundamental units of matter. They had assumed atoms were as small as anything could be and that they could not possibly be broken up into anything smaller. The discovery of the electron, however, had shown that some particles, at least, might be far smaller than any atom. Then, the investigations into radioactivity had shown that atoms of uranium and thorium spontaneously broke up into smaller particles, including electrons and alpha particles.
It would seem, then, that atoms of these elements and, presumably, of all elements, were made up of still smaller particles and that among these particles were electrons. The atom had a structure and physicists became interested in discovering exactly what that structure was.
The Structure of the Atom
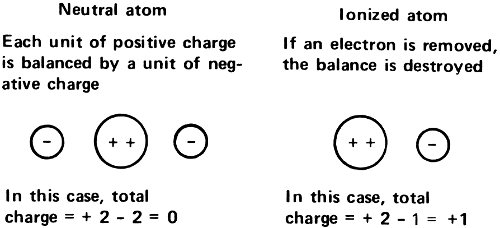
Since radioactive atoms gave off either positively charged particles or negatively charged particles, it seemed reasonable to assume that atoms generally were made up of both types of electricity. Furthermore, since the atoms in matter generally carried no charge at all, the normal “neutral atom” must be made up of equal quantities of positive charge and negative charge.
It turned out that only radioactive atoms, such as those of uranium and thorium, gave off positively charged alpha particles. Many atoms, however, that were not radioactive, could be made to give off electrons. In 1899 Thomson showed that certain perfectly normal metals with no trace of radioactivity gave off electrons when exposed to ultraviolet light. (This is called the “photoelectric effect”.)
It was possible to suppose, then, that the main structure of the atom was positively charged and generally immovable, and that there were also present light electrons, which could easily be detached. Thomson had suggested, as early as 1898, that the atom was a ball of matter carrying a positive charge and that individual electrons were stuck throughout its substance, like raisins in pound cake.
If something like the Thomson view were correct then the number of electrons, each with one unit of negative electricity, would depend on the total size of the positive charge carried by the atom. If the charge were +5, there would have to be 5 electrons present to balance that. The total charge would then be 0 and the atom as a whole would be electrically neutral.
If, in such a case, an electron were removed, the atomic charge of +5 would be balanced by only 4 electrons with a total charge of -4. In that case, the net charge of the atom as a whole would be +1. On the other hand, if an extra electron were forced onto the atom, the charge of +5 would be balanced by 6 electrons with a total charge of -6, and the net charge of the atom as a whole would be -1.
Such electrically charged atoms were called “ions” and their existence had been suspected since Faraday’s day. Faraday had known that atoms had to travel through a solution under the influence of an electric field to account for the way in which metals and gases appeared at the cathode and anode. It was he who first used the term, ion, from a Greek word meaning “traveller”. The word had been suggested to him by the English scholar, William Whewell (1794-1866). In 1884 the Swedish chemist Svante August Arrhenius (1859-1927) had first worked out a detailed theory based on the suggestion that these ions were atoms or groups of atoms that carried an electric charge.

Svante A. Arrhenius
By the close of the 19th century, then, Arrhenius’s suggestion seemed correct. There were positive ions made up of atoms or groups of atoms, from which one or more of the electrons within the atoms had been removed. There were negative ions made up of single atoms or of groups of atoms, to which one or more extra electrons had been added.
Although Thomson’s model of the atom explained the existence of ions and the fact that atoms could give off electrons or absorb them, it was not satisfactory in all ways. Further investigations yielded results not compatible with the raisins-in-the-pound-cake notion.
In 1906 Rutherford began to study what happened when massive subatomic particles, such as alpha particles, passed through matter. When alpha particles passed through a thin film of gold, for instance, they raced through, for the most part, as though nothing were there. The alpha particles seemed to push the light electrons aside and to act as though the positively charged main body of the atom that Thomson had pictured was not solid, but was soft and spongy.
The only trouble was that every once in a while an alpha particle seemed to strike something in the gold film and bounce to one side. Sometimes it even bounced directly backward. It was as though somewhere in each atom there was something at least as massive as the alpha particle.
How large was this massive portion of the atom? It couldn’t be very large for if it were the alpha particles would hit it frequently. Instead, the alpha particles made very few hits. This meant the massive portion was very small and that most alpha particles tore through the atom without coming anywhere near it.
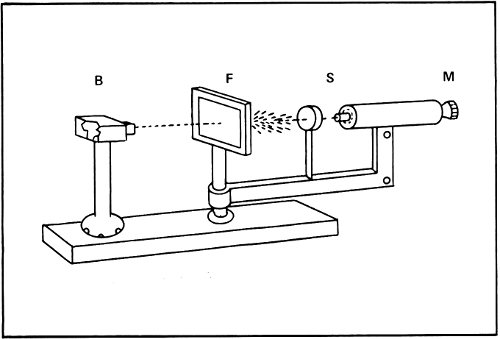
Rutherford’s alpha particle bombardment apparatus. A piece of radium in the lead box (B) emits alpha particles that go through the gold foil (F). These particles are scattered at different angles onto the fluorescent screen (S), where the flashes caused by each impact are seen through the microscope (M). Below, alpha particles are shown bouncing off a nucleus in the gold foil.
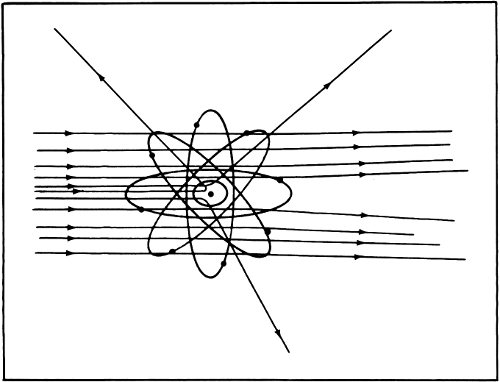
By 1911 Rutherford announced his results to the world. He suggested that just about all the mass of the atom was concentrated into a very tiny, positively charged “nucleus” at its center. The diameter of the nucleus was only about 1/10,000 the diameter of the atom. All the rest of the atom was filled with the very light electrons.
Hans Geiger (left) and Ernest Rutherford at Manchester University about 1910.
According to Rutherford’s notion, the atom consisted of a single tiny positively charged lead shot at the center of a foam of electrons. It was Thomson’s notion in reverse. Still, the nucleus carried a positive charge of a particular size and was balanced by negatively charged electrons. Rutherford’s 30 model of the atom explained the existence of ions just as easily as Thomson’s did and it explained more besides.
For instance, if all the electrons are removed so that only the nucleus remains, this nucleus is as massive as an atom but is so tiny in size that it can penetrate matter. The alpha particle would be a bare atomic nucleus from this point of view.
Rutherford’s model of the “nuclear atom” is still accepted today.
Atomic Numbers
Since the atom consisted of a positively charged nucleus at the center, and a number of negatively charged electrons outside, the next step was to find the exact size of the nuclear charge and the exact number of electrons for the different varieties of atoms.
The answer came through a line of research that began with the English physicist Charles Glover Barkla (1877-1944). In 1911 he noted that when X rays passed through atoms, some were absorbed and some bounced back. Those that bounced back had a certain ability to penetrate other matter. When the X rays struck atoms of high atomic weight, the X rays that bounced back were particularly penetrating. In fact, each different type of atom seemed associated with reflected X rays of a particular penetrating power, so Barkla called these “characteristic X rays”.
In 1913 another English physicist, Henry Gwyn-Jeffreys Moseley (1887-1915), went into the matter more thoroughly. He measured the exact wavelength of the characteristic X rays by reflecting them from certain crystals. In crystals, atoms are arranged in regular order and at known distances from each other. X rays reflecting from (or more accurately, diffracting from) crystals are bent out of their path by the rows of atoms. The longer their waves, the more they are bent. From the degree of bending the wavelength of the waves can be determined.

Charles Glover Barkla

Henry Gwyn-Jeffreys Moseley
Moseley found that the greater the atomic weight of an atom, the shorter the waves of the characteristic X rays associated with it and the more penetrating those X rays were. There was such a close connection, in fact, that Moseley could arrange the elements in order according to the wavelength of the characteristic X rays.
For some 40 years prior to this, the elements had been listed in order of atomic weight. This was useful especially since the Russian chemist Dmitri I. Mendeléev (1834-1907) had arranged them in a “periodic table” based on the atomic weight order in such a way that elements of similar properties were grouped together. The elements in this table were sometimes numbered consecutively (“atomic number”) but this was inconvenient since, when new elements were discovered, the list of atomic numbers might have to be reorganized.
Dmitri Mendeléev and Bohuslav Brauner in Prague in 1900. Brauner was a professor of chemistry at the Bohemian University in Prague.
The Danish physicist Niels Bohr (1885-1962) had just advanced a theory of atomic structure that made it reasonable to suppose that the wavelength of the characteristic X rays depended on the size of the nuclear charge of the atoms making up a particular element. Moseley therefore suggested that these X rays be used to determine the size of the positive charge on its nucleus. The atomic number could then be set equal to that charge and be made independent of new discoveries of elements.
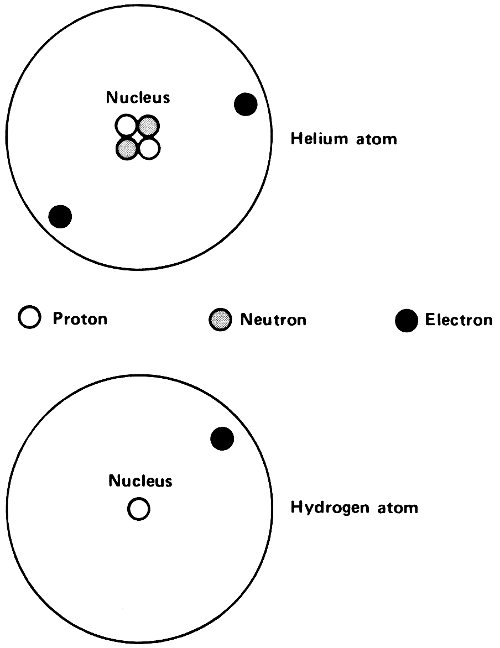
Hydrogen, for instance, has an atomic number of 1. Its nucleus carries a unit positive charge, +1, and the hydrogen atom possesses 1 electron to balance this. Helium, with an atomic number of 2, has a nuclear charge of +2 and 2 electrons, with a total charge of -2, to balance it. (The alpha particle released by radioactive atoms is identical with a helium nucleus.)
The atomic number increases as one goes up the line of atoms. Oxygen atoms, for instance, have an atomic number of 8 and iron atoms have one of 26. At the upper end, thorium is 90 and uranium is 92. Each uranium atom has a nucleus bearing a charge of +92 and contains 92 electrons to balance this.
Once the notion of the atomic number was worked out, it became possible to tell for certain whether any elements remained as yet undiscovered and, if so, where in the list they might be.
Thus, when Moseley first presented scientists with the atomic number it turned out that there were still 7 elements that were not discovered. At least elements with atomic numbers of 43, 61, 72, 75, 85, 87, and 91 were still not known. By 1945, all seven had been discovered.
It quickly turned out that the atomic number was more fundamental and more characteristic of a particular element than was the atomic weight.

Niels Bohr

Bohr’s study.
Since Dalton’s time it had been assumed that all the atoms of a particular element were of equal atomic weight and that atoms of two different elements were always of different atomic weight. The first inkling and the first proof that this might not be so came through the study of radioactivity.
Isotopes
In 1902 Rutherford and his co-worker Frederick Soddy (1877-1956) showed that when uranium atoms gave off alpha particles, a new kind of atom was formed that was not uranium at all. It was this new atom that was eventually found to give off a beta particle, and then another atom of still another element was formed. This work of Rutherford and Soddy began a line of investigation that by 1907 had shown that there was a whole radioactive chain of elements, each one breaking down to the next in line by giving off either an alpha particle or a beta particle, until finally a lead atom was formed that was not radioactive.

Frederick Soddy
There was, in short, a “radioactive series” beginning with uranium (atomic number 92) and ending with lead (atomic number 82). The same was true of thorium (atomic number 90), which began a series that also ended with lead. Still a third element, actinium (atomic number 89) was, at that time, the first known member of a series that also ended in lead.
The various atoms formed in these three radioactive series were not all different in every way. When the uranium atom gives off an alpha particle, it forms an atom originally called “uranium X₁”. On close investigation, it turned out that this uranium X₁ had the chemical properties of thorium. Uranium X₁, had, however, radioactive properties different from ordinary thorium.
Uranium X₁ broke down so rapidly, giving off beta particles as it did so, that half of any given quantity would have broken down in 24 days. Another way of saying this (which was introduced by Rutherford) was that the “half-life” of uranium X₁, is 24 days. Ordinary thorium, however, gives off alpha particles, not beta particles, and does so at such a slow rate, that its half-life is 14 billion years!
Uranium X₁, and ordinary thorium were in the same place in the list of elements by chemical standards, and yet there was clearly something different about the two.
Here is another case. In 1913 the British chemist Alexander Fleck (1889- ) studied “radium B” and “radium D”, the names given to two different kinds of atoms in the uranium radioactive series. He also studied “thorium B” in the thorium radioactive series and “actinium B” in the actinium radioactive series. All four are chemically the same as ordinary lead; all four are in the same place in the list of elements. Yet each is different from the radioactive standpoint. Though all give off beta particles, radium B has a 38 half-life of 27 minutes, radium D one of 19 years, thorium B one of 11 hours, and actinium B one of 36 minutes.
In 1913 Soddy called atoms that were in the same place in the list of elements, but which had different radioactive properties, “isotopes”, from Greek words meaning “same place”.
At first, it seemed that the only difference between isotopes might be in their radioactive properties and that only radioactive atoms were involved. Quickly that proved not to be so.
It proved that it was possible to have several forms of the same element that were all different even though none of them were radioactive. The uranium series, the thorium series, and the actinium series all ended in lead. In each case the lead formed was stable (not radioactive). Were the lead atoms identical in every case? Soddy had worked out the way in which atomic weights altered every time an alpha particle or a beta particle was given off by an atom. Working through the three radioactive series he decided that the lead atoms had different atomic weights in each case.
The uranium series ought to end with lead atoms that had an atomic weight of 206. The thorium series ought to end in lead atoms with an atomic weight of 208 and the actinium series in lead atoms with an atomic weight of 207.
If this were so, there would be 3 lead isotopes that would differ not in radioactive properties, but in atomic weight. The isotopes could be referred to as lead-206, lead-207, and lead-208. If we use the chemical symbol for lead (Pb), we could write the isotopes, ²⁰⁶Pb, ²⁰⁷Pb, and ²⁰⁸Pb. (We read the symbol ²⁰⁶Pb as lead-206.) Atomic weight measurements made in 1914 by Soddy and others supported that theory.
All 3 lead isotopes had the same atomic number of 82. The atoms of all 3 isotopes had nuclei with an electric charge of +82 and all 3 had 82 electrons in the atom to balance that positive nuclear charge. The difference was in the mass of the nucleus only.
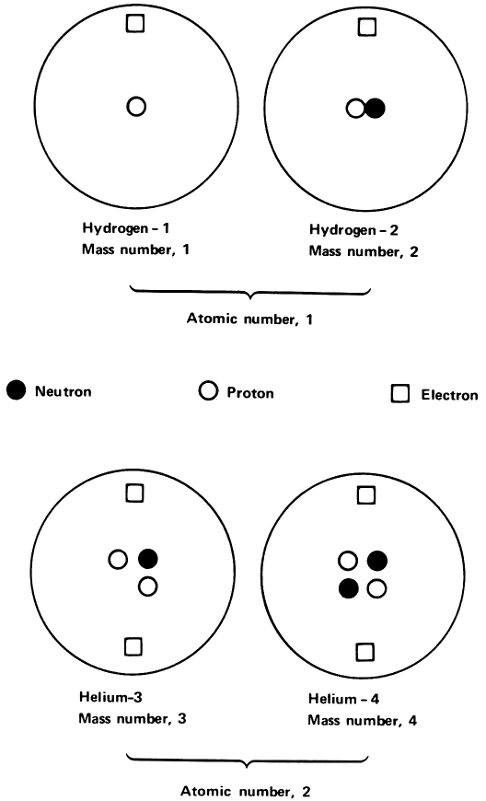
Isotopes of two elements.
But what of ordinary lead that existed in the rocks far removed from any radioactive substances and that had presumably been stable through all the history of earth? Its atomic weight was 207.2.
Was the stable lead that had no connection with radioactivity made up of atoms of still another isotope, one with a fractional atomic weight? Or could stable lead be made up of a mixture of isotopes, each of a different whole-number atomic weight and was the overall atomic weight a fraction only because it was an average?
It was at the moment difficult to tell in the case of lead, but an answer came in connection with another element, the rare gas neon (atomic symbol Ne), which has an atomic weight of 20.2.
Was that fractional atomic weight something that was possessed by all neon atoms without exception or was it the average of some lightweight atoms and some heavyweight ones? It would be a matter of crucial importance if isotopes of neon could be found, for neon had nothing to do with any of the radioactive series. If neon had isotopes then any element might have them.
In 1912 Thomson was working on neon. He sent a stream of cathode-ray electrons through neon gas. The electrons smashed into the neon atoms and knocked an electron off some of them. That left a neon ion carrying a single positive charge—an ion that could be written Ne⁺.
The neon ions move in the electric field as electrons do, but in the opposite direction since they have an opposite charge. In the combined presence of a magnet and of an electric field, the neon ions move in a curved path. If all the neon ions had the same mass, all would follow the same curve. If some were more massive than others, the more massive ones would curve less.
The neon ions ended on a photographic plate, which was darkened at the point of landing. There were two regions of 41 darkening, because there were neon ions of two different masses that curved in two different degrees and ended in two different places. Thomson showed, from the amount of curving, that there was a neon isotope with an atomic weight of 20 and one with an atomic weight of 22—²⁰Ne and ²²Ne.
What’s more, from the intensity of darkening, it could be seen that ordinary neon was made up of atoms that were roughly 90% ²⁰Ne and 10% ²²Ne. The overall atomic weight of neon, 20.2, was the average atomic weight of these 2 isotopes.
Thomson’s instrument was the first one capable of separating isotopes and such instruments came to be called “mass spectrometers”. The first to use the name was the English physicist Francis William Aston (1877-1945), who built the first efficient instrument of this type in 1919.
He used it to study as many elements as he could. He and those who followed him located many isotopes and determined the frequency of their occurrence with considerable precision. It turned out, for instance, that neon is actually 90.9% ²⁰Ne, and 8.8% ²²Ne. Very small quantities of still a third isotope, ²¹Ne, are also present, making up 0.3%.
As for ordinary lead in nonradioactive rocks, it is made up of 23.6% ²⁰⁶Pb, 22.6% ²⁰⁷Pb, and 52.3% ²⁰⁸Pb. There is still a fourth isotope, ²⁰⁴Pb, which makes up the remaining 1.5% and which is not the product of any radioactive series at all.
The isotopes always have atomic weights that are close to, but not quite, whole numbers. Any atomic weight of an element that departs appreciably from an integer does so only because it is an average of different isotopes. For instance, the atomic weight of chlorine (chemical symbol Cl) is 35.5, but this is because it is made up of a mixture of 2 isotopes. About one quarter of chlorine’s atoms are ³⁷Cl and about three-quarters are ³⁵Cl.

Francis W. Aston
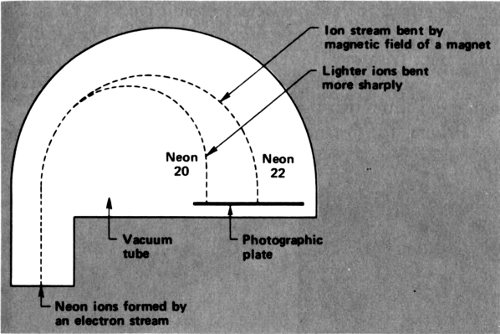
Mass spectrograph as used by Thomson and Aston to measure the atomic weight of neon.
To avoid confusion, the average mass of the isotopes that make up a particular element is still called the atomic weight of that element. The integer closest to the mass of the individual isotope is spoken of as the “mass number” of that isotope. Thus, chlorine is made up of isotopes with mass numbers 35 and 37, but the atomic weight of chlorine as it is found in nature is 35.5 (or, to be more accurate, 35.453).
In the same way, ordinary lead is made up of isotopes with mass numbers 204, 206, 207, and 208, and its atomic weight is 207.19; neon is made up of isotopes with mass numbers 20, 21, and 22, and its atomic weight is 20.183, and so on.
If the atomic weight of some element happens to be very close to a whole number to begin with, it may consist of a single kind of atom. For instance, the gas fluorine (chemical symbol F) has an atomic weight of nearly 19, while that of the metal sodium (chemical symbol Na) is nearly 23. As it turns out, all the atoms of fluorine are of the single variety ¹⁹F, while all the atoms of sodium are ²³Na.
Sometimes the atomic weight of an element, as it occurs in nature, is nearly a whole number and yet it is made up of more than 1 isotope. In that case, one of the isotopes makes up very nearly all of it, while the others are present in such minor quantities that the average is hardly affected.
Helium, for instance (atomic symbol He) has an atomic weight of just about 4 and, indeed, almost all the atoms making it up are ⁴He. However, 0.0001% of the atoms, or one out of a million, are ³He. Again, 99.6% of all the nitrogen atoms (atomic symbol N) are ¹⁴N, but 0.4% are ¹⁵N. Then, 98.9% of all carbon atoms (atomic symbol C) are ¹²C, but 1.1% are ¹³C. It is not surprising that the atomic weights of nitrogen and carbon are just about 14 and 12, respectively.

Harold Urey
Even hydrogen does not escape. Its atomic weight is just about 1 and most of its atoms are ¹H. The American chemist Harold Clayton Urey (1893- ) detected the existence of a 45 more massive isotope, ²H. This isotope has almost twice the mass of the lighter one. No other isotopes of a particular atom differ in mass by so large a factor. For that reason ²H and ¹H differ in ordinary chemical properties more than isotopes usually do and Urey therefore gave ²H the special name of “deuterium” from a Greek word meaning “second”.

W. F. Giauque
In 1929 the American chemist William Francis Giauque (1895- ) found that oxygen was composed of more than 1 isotope. Its atomic weight had been set arbitrarily at 16.0000 so it was a relief that 99.76% of its atoms were ¹⁶O. However, 0.20% were ¹⁸O, and 0.04% were ¹⁷O.
As you see, ¹⁶O must have a mass number of slightly less than 16.0000 and it must be the more massive isotopes ¹⁷O and ¹⁸O that pull the average up to 16.0000. Disregarding this, chemists clung to a standard atomic weight of 16.000 for oxygen as it appeared in nature, preferring not to concern themselves with the separate isotopes.
Physicists, however, felt uneasy at using an average as standard for they were more interested in working with individual isotopes. They preferred to set ¹⁶O at 16.0000 so that the average atomic weight of oxygen was 16.0044 and all other atomic weights rose in proportion. Atomic weights determined by this system were “physical atomic weights”.
Finally, in 1961, a compromise was struck. Chemists and physicists alike decided to consider the atomic weight of ¹²C as exactly 12 and to use that as a standard. By this system, the atomic weight of oxygen became 15.9994, which is only very slightly less than 16.
The radioactive elements did not escape this new view either. The atomic weight of uranium (chemical symbol U) is just about 238 and, indeed, most of its atoms are ²³⁸U. In 1935, however, the Canadian-American physicist, Arthur Jeffrey Dempster (1886-1950), found that 0.7% of its atoms were a lighter isotope, ²³⁵U.
These differed considerably in radioactive properties. The common uranium isotope, ²³⁸U, had a half-life of 4500 million years, while ²³⁵U had a half-life of only 700 million years. Furthermore ²³⁵U broke down in three stages to actinium. It was ²³⁵U, not actinium itself, that was the beginning of the actinium radioactive series.
As for thorium (atomic symbol Th) with an atomic weight of 232, it did indeed turn out that in the naturally occurring element virtually all the atoms were ²³²Th.
ENERGY
The Law of Conservation of Energy
We have now gone as far as we conveniently can in considering the intertwining strands of the atom and of electricity. It is time to turn to the third strand—energy.
To physicists the concept of “work” is that of exerting a force on a body and making it move through some distance. To lift a weight against the pull of gravity is work. To drive a nail into wood against the friction of its fibers is work.
Anything capable of performing work is said to possess “energy” from Greek words meaning “work within”. There are various forms of energy. Any moving mass possesses energy by virtue of its motion. That is, a moving hammer will drive a nail into wood, while the same hammer held motionlessly against the nailhead will not do so. Heat is a form of energy, since it will expand steam that will force wheels into motion that can then do work. Electricity, magnetism, sound, and light can be made to perform work and are forms of energy.
The forms of energy are so many and so various that scientists were eager to find some rule that covered them all and would therefore serve as a unifying bond. It did not seem impossible that such a rule might exist, since one had been found in connection with matter that appeared in even greater variety than energy did.
All matter, whatever its form and shape, possessed mass, and in the 1770s, the French chemist Antoine Laurent Lavoisier (1743-1794) discovered that the quantity of mass was constant. If a system of matter were isolated and made to undergo complicated chemical reactions, everything about it might change, but not its mass. A solid might turn into a gas; a single substance might change into two or three different substances, but whatever happened, the total mass at the end was exactly the same (as nearly as chemists could tell) as at the beginning. None was either created or destroyed, however, the nature of the matter might change. This was called the “law of conservation of mass”.

Lavoisier in his laboratory during his studies on respiration. From a sketch made by Madame Lavoisier.

Antoine Lavoisier and his wife.
Naturally, it would occur to scientists to wonder if a similar law might hold for energy. The answer wasn’t easy to get. It wasn’t as simple to measure the quantity of energy as it was to measure the quantity of mass. Nor was it as simple to pen up a quantity of energy and keep it from escaping or from gaining additional quantity from outside, as it was in the case of mass.
Beginning in 1840, however, the English physicist James Prescott Joule (1818-1889) began a series of experiments in which he made use of every form of energy he could think of. In each case he turned it into heat and allowed the heat to raise the temperature of a given quantity of water. He used the rise in temperature as a measure of the energy. By 1847 he was convinced that any form of energy could be turned into fixed and predictable amounts of heat; that a certain amount of work was equivalent to a certain amount of heat.
In that same year, the German physicist Hermann Ludwig Ferdinand von Helmholtz (1821-1894) advanced the general notion that a fixed amount of energy in one form was equal to the same amount of energy in any other form. Energy might change its form over and over, but not change its amount. None could either be destroyed or created. This is the “law of conservation of energy”.
Chemical Energy
There is energy in a piece of wood. Left quietly to itself, it seems completely incapable of bringing about any kind of work. Set it on fire, however, and the wood plus the oxygen in the air will give off heat and light that are clearly forms of energy. The heat could help boil water and run a steam engine.
The amount of energy in burning wood could be measured if it were mixed with air and allowed to burn in a closed container that was immersed in a known quantity of water. From the rise in temperature of the water, the quantity of energy produced could be measured in units called “calories” (from a Latin word for “heat”). The instrument was therefore called a “calorimeter”.
In the 1860s the French chemist Pierre Eugène Marcelin Berthelot (1827-1907) carried through hundreds of such determinations. His work and similar work by others made it clear that such “chemical energy”—the energy derived from chemical changes in matter—fit the law of conservation of energy.
Here’s how it looked in the last decades of the 19th century.
Molecules are composed of combinations of atoms. Within the molecules, the atoms stick together more or less tightly. It takes a certain amount of energy to pull a molecule apart into separate atoms against the resistance of the forces holding them together.
If, after being pulled apart, the atoms are allowed to come together again, they give off energy. The amount of energy they give off in coming together is exactly equal to the amount of energy they had to gain before they could separate.
This is true of all substances. For instance, hydrogen gas, as it is found on earth, is made up of molecules containing 2 hydrogen atoms each (H₂). Add a certain amount of energy and you pull the atoms apart; allow the atoms to come back together into paired molecules, and the added energy is given back again. The same is true for the oxygen molecule, which is made up of 2 oxygen atoms (O₂) and of the water molecule (H₂O). Always the amount of energy absorbed in one change is given off in the opposite change. The amount absorbed and the amount given off are always exactly equal.
However, the amount of energy involved differs from molecule to molecule. It is quite hard to pull hydrogen molecules apart, and it is even harder to pull oxygen molecules apart. You have to supply about 12% more energy to pull an oxygen molecule apart than to pull a hydrogen molecule apart. Naturally, if you let 2 oxygen atoms come together to form an oxygen molecule, you get back 12% more energy than if you allow 2 hydrogen atoms to come together to form a hydrogen molecule.
It takes a considerably larger amount of energy to pull apart a water molecule into separate atoms than to pull apart either hydrogen or oxygen molecules. Naturally, that greater energy is also returned once the hydrogen and oxygen atoms are allowed to come back together into water molecules.
Next, imagine pulling apart hydrogen and oxygen molecules into hydrogen and oxygen atoms and then having those atoms come together to form water molecules. A certain amount of energy is put into the system to break up the hydrogen and oxygen molecules, but then a much greater amount of energy is given off when the water molecules form.
It is for that reason that a great deal of energy (mostly in the form of heat) is given off if a jet of hydrogen gas and a jet of oxygen gas are allowed to mix in such a way as to form water.
Just mixing the hydrogen and oxygen isn’t enough. The molecules of hydrogen and oxygen must be separated and that takes a little energy. The energy in a match flame is enough to raise the temperature of the mixture and to make the hydrogen and oxygen molecules move about more rapidly and more energetically. This increases the chance that some molecules will be broken up into separate atoms (though the actual process is rather complicated). An oxygen atom might then strike a hydrogen molecule to form water (O + H₂ → H₂O) and more energy is given off than was 53 absorbed from the match flame. The temperature goes up still higher so that further breakup among the oxygen and hydrogen molecules is encouraged.
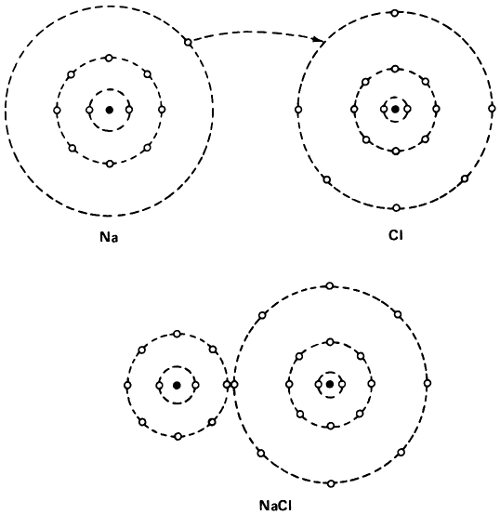
The formation of a sodium chloride molecule.
This happens over and over again so that in very little time, the temperature is very high and the hydrogen and oxygen are combining to form water at an enormous rate. If a great deal of hydrogen and oxygen are well-mixed to begin with, the rate of reaction is so great that an explosion occurs.
Such a situation, in which each reacting bit of the system adds energy to the system by its reaction and brings about more reactions like itself, is called a “chain reaction”. Thus, a match flame put to one corner of a large sheet of paper will set that corner burning. The heat of the burning will ignite a 54 neighboring portion of the sheet and so on till the entire sheet is burned. For that matter a single smoldering cigarette end can serve to burn down an entire forest in a vastly destructive chain reaction.
Electrons and Energy
The discovery of the structure of the atom sharpened the understanding of chemical energy.
In 1904 the German chemist Richard Abegg (1869-1910) first suggested that atoms were held together through the transfer of electrons from one atom to another.
To see how this worked, one began by noting that electrons in an atom existed in a series of shells. The innermost shell could hold only 2 electrons, the next 8, the next 18 and so on. It turned out that some electron arrangements were more stable than others. If only the innermost shell contained electrons and it were filled with the 2 electrons that were all it could hold, then that was a stable arrangement. If an atom contained electrons in more than one shell and the outermost shell that held electrons held 8, that was a stable arrangement, too.
Thus, the helium atom has 2 electrons only, filling the innermost shell, and that is so stable an arrangement that helium undergoes no chemical reactions at all. The neon atom has 10 electrons—2 in the innermost shell, and 8 in the next—and it does not react. The argon atom has 18 electrons—2, 8, and 8—and it too is very stable.
But what if an atom did not have its electron shell so neatly filled. The sodium atom has 11 electrons—2, 8, and 1—while the fluorine atom has 9 electrons—2 and 7. If the sodium atom passed one of its electrons to a fluorine atom, both would have the stable configuration of neon—2 and 8. This, therefore, ought to have a great tendency to happen.
If it did happen, though, the sodium atom, minus 1 electron, would have a unit positive charge and would be Na⁺, 55 a positively charged ion. Fluorine with 1 electron in excess would become F⁻, a negatively charged ion. The 2 ions, with opposite charges, would cling together, since opposite charges attract, and thus the molecule of sodium fluoride (NaF) would be formed.
In 1916 the American chemist Gilbert Newton Lewis (1875-1946) carried this notion farther. Atoms could cling together not only as a result of the outright transfer of 1 or more electrons, but through sharing pairs of electrons. This sharing could only take place if the atoms remained close neighbors, and it would take energy to pull them apart and break up the shared pool, just as it would take energy to pull 2 ions apart against the attraction of opposite charges.
In this way the vague notions of atoms clinging together in molecules and being forced apart gave way to a much more precise picture of electrons being transferred or shared. The electron shifts could be dealt with mathematically by a system that came to be called “quantum mechanics” and chemistry was thus made a more exact science than it had ever been before.
The Energy of the Sun
The most serious problem raised by the law of conservation of energy involved the sun. Until 1847, scientists did not question sunlight. The sun radiated vast quantities of energy but that apparently was its nature and was no more to be puzzled over than the fact that the earth rotated on its axis.
Once Helmholtz had stated that energy could neither be created nor destroyed, however, he was bound to ask where the sun’s energy came from. It had, to man’s best knowledge, been radiating heat and light, with no perceptible change, throughout the history of civilization and, from what biologists and geologists could deduce, for countless ages earlier. Where, then, did that energy come from?
The sun gave the appearance of being a huge globe of fire. Could it actually be that—a large heap of burning fuel, turning chemical energy into heat and light?
The sun’s mass was known and its rate of energy production was known. Suppose the sun’s mass were a mixture of hydrogen and oxygen and it were burning at a rate sufficient to produce the energy at the rate it was giving it off. If that were so, all the hydrogen and oxygen in its mass would be consumed in 1500 years. No chemical reaction in the sun could account for its having given us heat and light since the days of the pyramids, let alone since the days of the dinosaurs.
Was there some source of energy greater than chemical energy? What about the energy of motion? Helmholtz suggested that meteors might be falling into the sun at a steady rate. The energy of their collisions might then be converted into heat and light and this could keep the sun shining for as long as the supply of meteors held out—even millions of years.
This, however, would mean that the sun’s mass would be increasing steadily, and so would the force of its gravitational pull. With the sun’s gravitational field increasing steadily, the length of earth’s year would be decreasing at a measurable rate—but it wasn’t.
In 1854 Helmholtz came up with something better. He suggested that the sun was contracting. Its outermost layers were falling inward, and the energy of this fall was converted into heat and light. What’s more, this energy would be obtained without any change in the mass of the sun whatever.
Helmholtz calculated that the sun’s contraction over the 6000 years of recorded history would have reduced its diameter only 560 miles—a change that would not have been noticeable to the unaided eye. Since the development of the telescope, two and a half centuries earlier, the decrease in 57 diameter would have been only 23 miles and that was not measurable by the best techniques of Helmholtz’s day.
Working backward, however, it seemed that 25 million years ago, the sun must have been so large as to fill the earth’s orbit. Clearly the earth could not then have existed. In that case, the maximum age of the earth was only 25 million years.
Geologists and biologists found themselves disturbed by this. The slow changes in the earth’s crust and in the evolution of life made it seem very likely that the earth must have been in existence—with the sun delivering heat and light very much in the present fashion—for many hundreds of millions of years.
Yet there seemed absolutely no other way of accounting for the sun’s energy supply. Either the law of conservation of energy was wrong (which seemed unlikely), or the painfully collected evidence of geologists and biologists was wrong (which seemed unlikely),—or there was some source of energy greater than any known in the 19th century, whose existence had somehow escaped mankind (which also seemed unlikely).
Yet one of those unlikely alternatives would have to be true. And then in 1896 came the discovery of radioactivity.
The Energy of Radioactivity
It eventually became clear that radioactivity involved the giving off of energy. Uranium emitted gamma rays that we now know to be a hundred thousand times as energetic as ordinary light rays. What’s more, alpha particles were being emitted at velocities of perhaps 30,000 kilometers per second, while the lighter beta particles were being shot off at velocities of up to 250,000 kilometers per second (about 0.8 times the velocity of light).
At first, the total energy given off by radioactive substances seemed so small that there was no use worrying 58 about it. The amount of energy liberated by a gram of uranium in 1 second of radioactivity was an insignificant fraction of the energy released by a burning candle.
In a few years, however, something became apparent. A lump of uranium might give off very little energy in a second, but it kept on for second after second, day after day, month after month, and year after year with no perceptible decrease. The energy released by the uranium over a very long time grew to be enormous. It eventually turned out that while the rate at which uranium delivered energy did decline, it did so with such unbelievable slowness that it took 4.5 billion years (!) for that rate to decrease to half what it was to begin with.
If all the energy delivered by a gram of uranium in the course of its radioactivity over many billions of years was totalled, it was enormously greater than the energy produced by the burning of a candle with a mass equal to that of uranium.
Let’s put it another way. We might think of a single uranium atom breaking down and shooting off an alpha particle. We might also think of a single carbon atom combining with 2 oxygen atoms to form carbon dioxide. The uranium atom would give off 2,000,000 times as much energy in breaking down, as the carbon atom would in combining.
The energy of radioactivity is millions of times as intense as the energy released by chemical reactions. The reason mankind had remained unaware of radioactivity and very aware of chemical reactions was, first, that the most common radioactive processes are so slow that their great energies were stretched over such enormous blocks of time as to be insignificant on a per second basis.
Secondly, chemical reactions are easily controlled by changing quantities, concentrations, temperatures, pressures, states of mixtures, and so on, and this makes them easy to 59 take note of and to study. The rate of radioactive changes, however, could not apparently be altered. The early investigators quickly found that the breakdown of uranium-238, for instance, could not be hastened by heat, pressure, changes in chemical combination, or, indeed, anything else they could think of. It remained incredibly slow.
But despite all this, radioactivity had at last been discovered and the intensity of its energies was recognized and pointed out in 1902 by Marie Curie and her husband Pierre Curie (1859-1906).
Where, then, did the energy come from? Could it come from the outside? Could the radioactive atoms somehow collect energy from their surroundings, concentrate it several million-fold, and then let it out all at once?
To concentrate energy in this fashion would violate something called “the second law of thermodynamics”. This was first proposed in 1850 by the German physicist Rudolf Julius Emmanuel Clausius (1822-1888) and had proved so useful that physicists did not like to abandon it unless they absolutely had to.
Another possibility was that radioactive atoms were creating energy out of nothing. This, of course, violated the law of conservation of energy (also called “the first law of thermodynamics”) and physicists preferred not to do that either.
The only thing that seemed to remain was to suppose that somewhere within the atom was a source of energy that had never made itself evident to humanity until the discovery of radioactivity. Becquerel was one of the first to suggest this.
It might have seemed at first that only radioactive elements had this supply of energy somewhere within the atom, but in 1903 Rutherford suggested that all atoms had a vast energy supply hidden within themselves. The supply in uranium and thorium leaked slightly, so to speak, and that was all that made them different.
The room in which the Curies discovered radium. Pierre Curie’s writing is on the blackboard.
But if a vast supply of energy existed in atoms, it was possible that the solution to the puzzle of the sun’s energy might rest there. As early as 1899 the American geologist Thomas Chrowder Chamberlin (1843-1928) was already speculating about a possible connection between radioactivity and the sun’s energy.
If it were some variety of this newly discovered source of energy (not necessarily ordinary radioactivity, of course) that powered the sun—millions of times as intense as chemical energy—then the sun might be pouring out energy for hundreds of millions of years without perceptible physical change—just as uranium would show scarcely any change even in so mighty a time span. The sun would not have to be contracting; it would not have had to fill the earth’s orbit 25,000,000 years ago.
This was all exciting, but in 1900 the structure of the atom had not yet been worked out and this new energy was just a vague supposition. No one had any idea of what it actually might be or where in the atom it might be located. It could only be spoken of as existing “within the atom” and was therefore called “atomic energy”. Through long habit, it is still called that much of the time. And yet “atomic energy” is not a good name. In the first couple of decades of the 20th century, it became apparent that ordinary chemical energy involved electron shifts and those electrons were certainly components of atoms. This meant that a wood fire was a kind of atomic energy.
The electrons, however, existed only in the outer regions of the atom. Once Rutherford worked out the theory of the nuclear atom, it became apparent that the energy involved in radioactivity and in solar radiation had to involve components of the atom that were more massive and more energetic than the light electrons. The energy had to come, somehow, from the atomic nucleus.
What is involved then in radioactivity and in the sun is “nuclear energy”. That is the proper name for it and in the 62 next section we will consider the subsequent history of the nuclear energy that broke upon the startled consciousness of scientists as the 20th century opened and which, less than half a century later, was to face mankind with untold consequences for good and for evil.
FOOTNOTES
QUOTATION CREDIT
| Inside front cover | Copyright © by Abelard-Shuman, Ltd., New York. Reprinted by permission from Inside the Atom, Isaac Asimov, 1966. |
PHOTO CREDITS
| Cover | The Metropolitan Museum of Art |
| Page facing inside cover | The “Horsehead” Nebula in Orion. Hale Observatories. |
| Author’s Photo | Jay K. Klein |
| Contents page & page 4 | Lick Observatory |
| Page | |
| 7 | New York Public Library |
| 9 | From Discovery of the Elements, Mary E. Weeks, Chemical Education Publishing Company, 1968. |
| 12 | Library of Congress |
| 15 | Sir George Thomson |
| 18 | Burndy Library |
| 19 | New York Public Library |
| 21 | Copyright © 1965 by Barbara Lovett Cline, reprinted from her volume The Questioners: Physicists and the Quantum Theory by permission of Thomas Y. Crowell Company, Inc., New York. |
| 22 & 23 | Curie Foundation, Institute of Radium |
| 26 | Academic Press, Inc. |
| 29 | Van Nostrand Reinhold Company |
| 31 | Top, Nobel Institute; bottom, from Discovery of the Elements, Mary E. Weeks, Chemical Education Publishing Company, 1968. |
| 32 | From Discovery of the Elements, Mary E. Weeks, Chemical Education Publishing Company, 1968. |
| 34 | Top, Nobel Institute; bottom, Niels Bohr Institute. |
| 36, 42, 44, & 45 | Nobel Institute |
| 48 | Academic Press, Inc. |
| 49 | From Discovery of the Elements, Mary E. Weeks, Chemical Education Publishing Company, 1968. |
| 60 | Curie Foundation, Institute of Radium |
★ U.S. GOVERNMENT PRINTING OFFICE: 1975—640—285/13

