.

OUR ATOMIC WORLD
by C. Jackson Craven
THE STORY OF ATOMIC ENERGY
U.S. ATOMIC ENERGY COMMISSION
Division of Technical Information
Understanding the Atom Series
The Understanding the Atom Series
Nuclear energy is playing a vital role in the life of every man, woman, and child in the United States today. In the years ahead it will affect increasingly all the peoples of the earth. It is essential that all Americans gain an understanding of this vital force if they are to discharge thoughtfully their responsibilities as citizens and if they are to realize fully the myriad benefits that nuclear energy offers them.
The United States Atomic Energy Commission provides this booklet to help you achieve such understanding.

Edward J. Brunenkant, Director
Division of Technical Information
UNITED STATES ATOMIC ENERGY COMMISSION
Dr. Glenn T. Seaborg, Chairman
James T. Ramey
Wilfrid E. Johnson
Dr. Theos J. Thompson
Dr. Clarence E. Larson
OUR ATOMIC WORLD
by C. Jackson Craven
CONTENTS
- THE GREEKS WERE CURIOUS ABOUT MATTER 1
- THE ATOMIC THEORY IS CONFIRMED 2
- CATHODE RAYS SHOW ATOMS CONTAIN SMALLER PARTS 3
- RADIOACTIVE ATOMS DISCOVERED 5
- RUTHERFORD FINDS THE ATOMIC NUCLEUS 6
- THE PROTON IS RECOGNIZED 8
- ISOTOPES ARE DISCOVERED 9
- THE ALCHEMISTS’ DREAM COMES TRUE 10
- SOME PARTICLES HAVE NO ELECTRIC CHARGE 13
- MATTER IS ENERGY; ENERGY IS MATTER 14
- NUCLEI CONTAIN ENERGY 15
- CHRONOLOGY 18
- FISSION IS EXPLAINED 20
- THE FISSION BOMB IS EXPLODED 23
- NUCLEAR ENERGY IS NEEDED FOR THE FUTURE 25
- FUSION HAS POTENTIAL 26
- ISOTOPES HAVE MANY USES 29
- RADIOISOTOPES AT WORK 30
- THE ATOMIC ENERGY COMMISSION 31
- TOWARD AN INTERNATIONAL ATOM 33
- SUGGESTED REFERENCES 35
United States Atomic Energy Commission
Division of Technical Information
Library of Congress Catalog Card Number: 63-64918
1963; 1964 (Rev.)
The cover is a time-exposed photograph of an animated model of a uranium-235 atom. The center represents the nucleus, greatly exaggerated in size. The fine lines represent the electrons whirling about the nucleus.
Courtesy Union Carbide Corporation
C. JACKSON CRAVEN is a teacher’s teacher as well as a student’s teacher, and has had an active career aiding understanding of atomic energy as a member of the University of Tennessee faculty and on the staff of the Oak Ridge Institute of Nuclear Studies. He has conducted short courses to instruct groups of high school science teachers in nuclear energy, and has served in a key capacity in training Institute demonstration-lecturers who visit high schools throughout the nation.
Dr. Craven worked during World War II for the Manhattan Project, which built the first atomic bomb. He earned bachelor’s and graduate degrees at the University of North Carolina, and later taught physics and mathematics at Delta State Teachers College and at Furman and Emory Universities.
His research interests include infrared spectroscopy, gaseous diffusion through porous media, and the physical properties of fibers.
OUR ATOMIC WORLD
By C. Jackson Craven
The story of atomic energy evolves from the curiosity of people concerning the nature and structure of matter, the stuff of which all material things are made.
The Greeks Were Curious About Matter
Certain philosophers of ancient Greece—Democritus for one—were fascinated by the question: what is matter? You can imagine one of the philosophers saying to his pupils:
“Gentlemen, let us consider a piece of cheese. With a knife we can cut it in two, thus obtaining smaller pieces. We can then cut one of these smaller pieces in two, obtaining still smaller pieces. We can think about repeating this process over and over to get smaller and smaller pieces of cheese. Now can this process be continued without limit, or will a time come when we arrive at the smallest possible piece of cheese? In other words, is there a piece so small that we must have at least that much or none, with no choice in between?”
It is probable that most people who thought about this question at all during the next two thousand years answered the last question in the negative. The prevailing notion was that matter was continuous, with no theoretical limit as to how small a piece of cheese, or anything else, might be.
This concept was humorously expressed by the British mathematician Augustus De Morgan (1806-1871) in these lines:
Great fleas have little fleas upon their backs to bite ’em,
And little fleas have lesser fleas, and so, ad infinitum.
The Atomic Theory Is Confirmed
De Morgan evidently did not keep up with the latest developments in science, however, because two years before his birth, John Dalton, an English schoolteacher, had changed the atomic theory of matter from a philosophical speculation into a firmly established principle. The evidence that convinced Dalton and many other contemporary scientists of the reality of atoms came from quantitative chemical analysis.
Dalton knew that many chemical substances could be separated into two or more simpler substances. Chemicals that could be separated further were called compounds; those that could not were called elements. Careful experiments by Dalton and others showed that whenever two or more elements combined chemically to make a compound the relative amounts of the elements had to be carefully adjusted to fit a definite proportion in order to have no elements left over after the reaction was finished. For example, if hydrogen and oxygen were combined to form water, the weight of oxygen had to be eight times the weight of hydrogen; otherwise, either some hydrogen or some oxygen would be left over.
This fundamental truth is now called the Law of Definite Proportions. Another important principle, called the Law of Multiple Proportions, is illustrated by hydrogen peroxide, which is made up of the same two elements that are found in water. The weight of oxygen in hydrogen peroxide, however, is 16 times the weight of hydrogen or exactly twice the relative weight found in water.
These principles of chemical combination convinced Dalton that each chemical element consists of small, 3 indivisible units, all just alike, called atoms, and that each chemical compound also has basic units, called molecules, which cannot be divided without reducing the compound into its elements—that is, destroying it as a compound. He visualized a molecule of a compound as formed by the uniting of individual atoms of two or more elements. It was obvious to him that in any molecule of a compound, the weight of each atom of a component element bore a proportionate relationship to the weight of the entire molecule which was equal to the proportion, by weight, of all that element in the compound. And although Dalton had no idea how heavy any individual atom really was, he could tell how many times heavier or lighter it was than an atom of another element.
Incidentally, Dalton mistakenly thought that one atom of oxygen was eight times as heavy as one atom of hydrogen instead of 16 times as heavy. He assumed a water molecule to be HO instead of H₂O.
Cathode Rays Show Atoms Contain Smaller Parts
Curiosity about the fundamental nature of matter was matched by equally avid curiosity about the fundamental nature of electricity. Before 1850 much had been learned about the behavior of electric charge and electric currents flowing through solids and liquids. Real progress in understanding electric charge, however, had to wait for the development of highly efficient vacuum pumps.
About 1854 Heinrich Geissler, a German glassblower, developed an improved suction pump, and also succeeded in sealing into a glass tube two wires attached to metal electrodes inside the tube. Experimenters were then able to study the flow of electricity through a near-vacuum. A Geissler tube is diagramed in Figure 1.
By the 1890s it had become clear that the flow of electricity through a highly evacuated tube consisted of a negative electric charge moving at a very high speed along straight lines between sealed-in electrodes. Since it originated at the negative electrode, or cathode, the invisible stream of charge was named “cathode rays.”
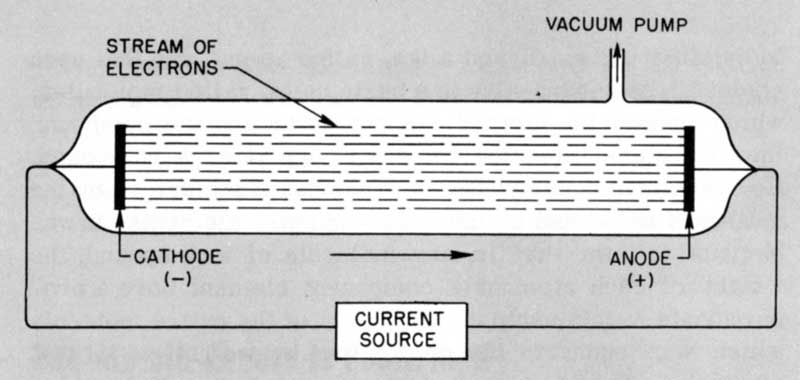
Figure 1 Geissler Tube.
- CURRENT SOURCE
- CATHODE (-)
- STREAM OF ELECTRONS
- VACUUM PUMP
- ANODE (+)
Although many investigators contributed to knowledge about cathode rays, the experiments of Joseph J. Thomson, a British physicist, are generally considered to have been the most enlightening. Thomson arranged a cathode-ray tube so that the rays could be deflected by magnets and by electrically charged metal plates. By applying certain well-known principles of physics, he was able to confirm an impression already held by physical chemists, namely, that electric charge, like matter, was “atomized”—the stream of charge consisted of a swarm of very small particles, all alike. He succeeded also in determining that the speed of the particles was about one-tenth the speed of light.
Probably Thomson’s most significant result was determining the ratio of the charge of each little particle to its weight. He was able to do this by measuring the magnetic force required to divert a stream of charged particles. (You can do this experiment yourself with relatively simple equipment.) This charge-to-weight ratio proved to be nearly 2000 times greater than the already known charge-to-weight ratio for a positively charged hydrogen atom, or ion, which until then was thought to be the lightest constituent of matter. It remained to be determined whether charge or weight caused the difference. Further experimentation showed that the charges were approximately the same amount in the two cases. It was therefore proven that the weight of the hydrogen atom, lightest of all the atoms, was nearly 2000 times as great as the weight of one of the little negative particles.
The name “electron” was given to the small negative particles identified by Thomson. Since the electrons had come from the cathode, it was apparent that the atoms in the cathode must contain electrons. Thomson reasoned that electric current in a wire is a stream of electrons passing successively from atom to atom and that the difference between an electrically charged atom and a neutral atom is that the charged one has gained or lost one or more electrons.
Radioactive Atoms Discovered

Henri Becquerel
Courtesy Journal of Chemical Education, Discovery of the Elements, Mary Elvira Weeks.
In 1896 the French physicist Henri Becquerel was investigating the relation between fluorescence and X rays, a puzzling kind of penetrating radiation discovered a few months earlier by the German, Wilhelm Roentgen. Various chemical compounds fluoresce, or glow, when exposed to ultraviolet rays and other types of radiation. While experimenting with a large number of chemicals, Becquerel discovered, quite by accident, that a compound containing the element uranium can, without being exposed to any kind of radiation, darken a photographic plate completely wrapped in heavy black paper.
Although no one realized it at the time, Becquerel had discovered that atoms of some elements will at random times transform themselves into atoms of a different element by emitting certain extremely high-speed charged particles. Atoms that can do this are said to be radioactive, and it was the radiation from transforming uranium atoms that darkened Becquerel’s photographic plate.
Rutherford Finds the Atomic Nucleus

Ernest Rutherford, 1871-1937
Courtesy Nobelstiftelsen
We are greatly indebted to the imagination and experimental skill of the British physicist Ernest Rutherford for the interpretation of radioactivity in terms of the structure of atoms.
Rutherford, born and educated in New Zealand, moved to England to work under Thomson at Cambridge University in 1895. Shortly afterward, Wilhelm Roentgen in Germany discovered X rays, Becquerel in France discovered radioactivity, and Thomson proved the existence of the electron.
During the next few years, curiosity about the fundamental nature of radioactivity led a number of people to do a great deal of work. The element thorium was found to be radioactive, and Marie and Pierre Curie discovered two new elements, polonium and radium, that were also radioactive. The radiation from radioactive materials was found to be of three kinds called alpha rays, beta rays, and gamma rays. Alpha rays were first detected by Rutherford, who later identified them as positively charged helium atoms. Becquerel demonstrated that beta rays, like cathode rays, consist of negatively charged electrons. The highly penetrating gamma rays were proved by Rutherford and E. N. da C. Andrade to be electromagnetic radiation similar to X rays.
Rutherford, in collaboration with the English chemist Frederick Soddy, brought order out of a chaos of puzzling discoveries by establishing the general behavior of radioactive atoms. He determined that certain naturally occurring atoms of high atomic weight can spontaneously emit an alpha or a beta particle and thereby convert themselves 7 into new atoms. These new atoms, being also radioactive, sooner or later convert themselves into still different atoms, and so on. Each time an alpha particle is emitted in this sequence, the new atom is lighter by the weight of the alpha particle, or helium atom. The disintegration process proceeds from stage to stage until at last a stable atom is produced. The end product in this “decay” process in naturally occurring radioactive elements is lead.
One experiment by Rutherford and his co-workers had a most profound effect on the understanding of atomic structure. What they did was to direct a stream of alpha particles at a thin piece of gold foil. The results were astonishing. Almost all the particles passed straight through the foil without changing direction. Of the few particles that did ricochet in new directions, however, some were deflected at very sharp angles. (See Figure 2.)
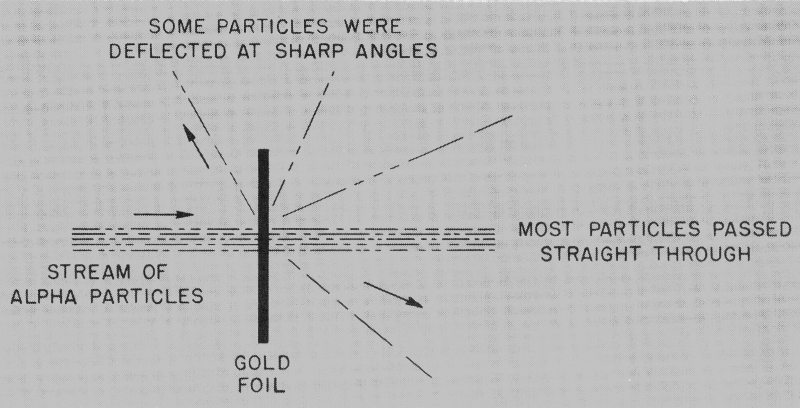
Figure 2 Rutherford’s most famous experiment, which led him to the concept of the nucleus.
As a result of this experiment, Rutherford proposed a concept of the atom entirely different from the one which prevailed at this time. The prevailing notion was one advanced by Thomson which conceived of an atom as a blob of positive electric charge in which were imbedded, in much the same way as plums are in a pudding, enough electrons to neutralize the positive charge. Rutherford’s concept, which quickly set aside Thomson’s “plum pudding” model, was that an atom has all of its positive charge and virtually all of its mass concentrated in a tiny space at its center. 8 (Collisions with this center, which came to be known thereafter as the nucleus, had been responsible for the sharp changes in direction of some of the alpha particles.) The space surrounding this nucleus is entirely empty except for the presence of a number of electrons (79 in the case of the gold atom), each about the same size as the nucleus.
To illustrate Rutherford’s concept, let us imagine a gold atom magnified so that it is as large as a bale of cotton. The nucleus at the center of this large atom would be the size of a speck of black pepper. If this imaginary bale weighed 500 pounds, the little speck at its center would weigh 499¾ pounds; the surrounding cotton (corresponding to empty space in Rutherford’s concept) containing the 79 electrons would weigh but ¼ pound. To express this idea another way, any object such as a gold ring, as dense and solid as it may seem to us, consists almost entirely of nothing!
The Proton Is Recognized
Rutherford’s discovery aroused intense curiosity about the nature and possible structure of this extremely small, but all-important, part of an atom. It was assumed that the positive charge carried by the nucleus must be a whole-number multiple of a small unit equal in size but opposite in sign to the charge of an electron. This conclusion was based on the information that all atoms contain electrons and that an undisturbed atom is electrically neutral. Since it was known that a neutral atom of hydrogen contains just one electron, it appeared that the charge on a hydrogen nucleus must represent the fundamental unit of positive charge, some multiple of which would represent the charge on any other nucleus. Several lines of investigation combined to establish quite firmly that nuclei of atoms occupying adjacent positions on the periodic chart of the elements differed in charge by this fundamental unit. Since the hydrogen nucleus seemed to play such an important role in making up the charges of all other nuclei, it was given the name proton from the Greek “protos,” which means “first.”
Isotopes Are Discovered
At a historic meeting of the British Association for the Advancement of Science held in Birmingham, England, in 1913, two apparently unrelated lines of investigation were reported, each of which showed that some atomic nuclei have identical electric charges but different weights.
One report was presented by Frederick Soddy, who had collaborated with Rutherford in explaining the pattern of natural radioactivity. Soddy knew that the nucleus of a radioactive atom loses both weight and positive charge when it throws out an alpha particle (helium nucleus). On the other hand, when a nucleus emits a beta particle (negative electron), its positive charge increases, but its weight is practically unchanged. Thus Soddy could deduce the weights and nuclear charges of many radioactive products. In several cases the products of two different kinds of radioactivity had the same nuclear charge but different weights. Since it is the positive charge carried by the nucleus of an atom which fixes the number of negative electrons needed to complete the atom, the nuclear charge is really responsible for the exterior appearance, or chemical properties, of the atom.
This conclusion was confirmed by unsuccessful efforts to separate by chemical means different radioactive products having the same nuclear charge but different weights. The products might have had quite different rates of radioactive disintegration, but they appeared to consist of chemically identical atoms of the same chemical element and hence to belong at the same place on the periodic chart of the elements. Soddy suggested that such atoms be called isotopes, from a Greek word meaning “same place.”
At the same meeting, Francis W. Aston, an assistant of Thomson, described what happened when charged atoms, or ions, of neon gas were accelerated in a discharge tube similar to the cathode-ray tube in which Thomson had discovered the electron. The rapidly moving neon ions were deflected by a magnet. Since light objects are more easily deflected than heavy objects, the amount of deflection indicated the weight. By making a comparison with a familiar gas like oxygen, Thomson and Aston were actually 10 able to measure the atomic weight of neon. To their surprise they found two kinds of neon. About nine-tenths of the neon atoms had an atomic weight of 20, and the remainder an atomic weight of 22.
What Thomson and Aston had done was to show that the stable element neon is a mixture of two isotopes. A device that can do what their apparatus did is called a mass spectrograph. (See Figure 3.) Since their time, instruments of this type have shown that more than three-fourths of the stable chemical elements are mixtures of two or more stable isotopes; in fact, there are about 300 such isotopes in all. The number of known unstable radioactive isotopes (radioisotopes), natural or man-made, is greater than 1000 and is still growing!
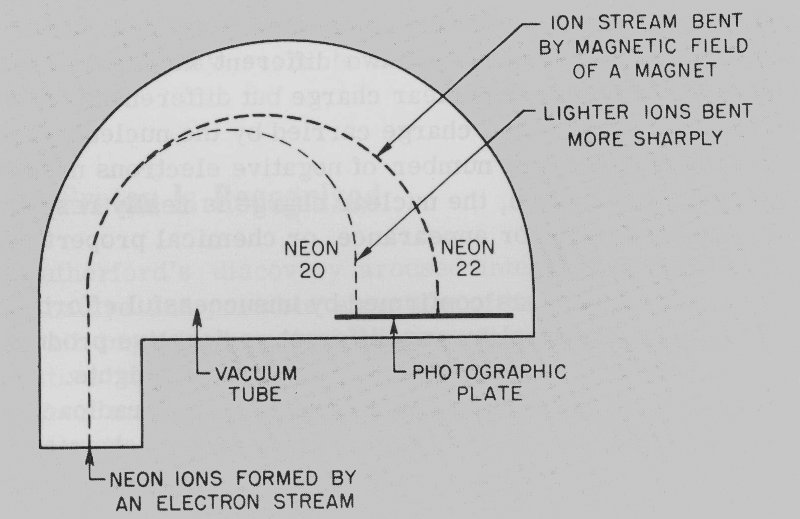
Figure 3 Mass spectrograph as used by Thomson and Aston to measure the atomic weight of neon.
- NEON 20
- NEON 22
The Alchemists’ Dream Comes True
During the Middle Ages the desire to find a way to convert a base metal like lead into gold was the outstanding incentive for research in chemistry. When the important role of the nucleus in determining the chemical properties of an atom became clear and the natural transmutation accompanying 11 radioactivity was understood, the fascinating idea occurred to many people that perhaps man would soon be able to alter the nucleus of a stable atom and thus deliberately convert one element into another. In a historic lecture delivered in Washington, D. C., in April 1914, Rutherford said, “It is possible that the nucleus of an atom may be altered by direct collision of the nucleus with very swift electrons or atoms of helium (i.e., beta or alpha particles) such as are ejected from radioactive matter.... Under favorable conditions, these particles must pass very close to the nucleus and may either lead to a disruption of the nucleus or to a combination with it.”

Medieval Alchemist
Courtesy Fisher Scientific Company
World War I began shortly after Rutherford made this statement, and preoccupation with war work stopped his experiments with nuclei. In 1919, however, he published a paper describing what happens when alpha particles pass through nitrogen gas. Very fast protons, or hydrogen nuclei, appear to originate along the paths of the alpha particles. The following is from Rutherford’s paper:
“If this be the case, we must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle, and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus.... The results as a whole suggest that, if alpha particles or similar projectiles of still greater energy were available for experiment, we might expect to break down the nuclear structure of many of the lighter atoms.”
This prediction has certainly been verified through the use of the atomic artillery provided by extremely powerful particle accelerators, or “atom smashers.”[1]
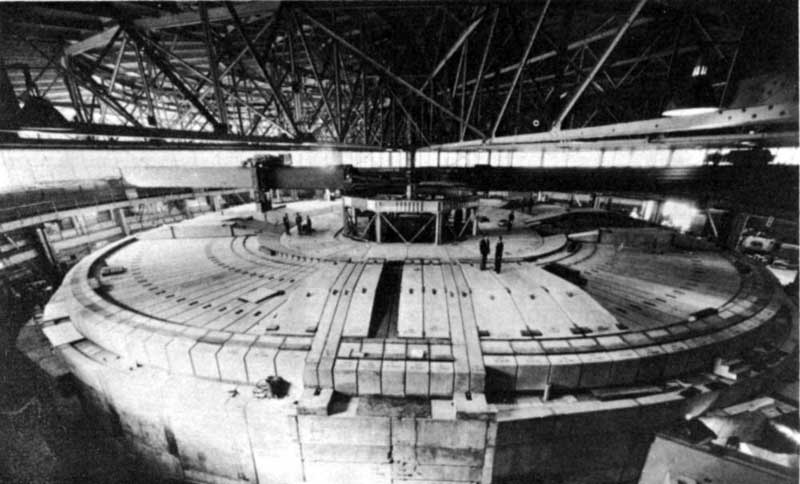
The Bevatron accelerator at the University of California’s Lawrence Radiation Laboratory, Berkeley, California, shown after recent remodeling in which it was enclosed in concrete shielding.
Courtesy Lawrence Radiation Laboratory
Patrick Blackett in England and W. D. Harkins in the United States soon proved independently that, during the nuclear event reported by Rutherford in his 1919 paper, an alpha particle combines with a nitrogen nucleus and that the resulting unstable combination immediately emits a proton and ends up as one of the isotopes of oxygen. This was the first instance of deliberate transmutation of one stable chemical element into another. Since that time practically every known element has been transmuted by bombardment. The dream of the alchemists has been partially fulfilled in that mercury has been changed into gold. We say “partially fulfilled” because the process is much too expensive to be economically profitable.
Some Particles Have No Electric Charge
During the early 1920s a number of investigators, including Harkins in the United States, Orme Masson in Australia, and Rutherford and his assistant James Chadwick in England, seriously considered the possibility that a neutral particle might exist in nature, possibly formed by the very close association of a proton and an electron. However, strenuous efforts to produce such particles by combining protons and electrons were unsuccessful.
During these years the new technique of bombarding all kinds of matter with alpha particles to see what would happen was widely exploited, and it gradually became clear that in a few instances a peculiar and highly penetrating kind of radiation was produced. In 1932, Chadwick succeeded in showing that the peculiar radiation must consist of a stream of particles, each weighing about the same as a proton but having no electrical charge.
The name “neutron” for a possible neutral particle of this type was suggested by Harkins in the United States in 1921. Much evidence now exists that the neutron is a fundamental particle in its own right and that it should not be thought of merely as a particle formed by a very close association between a proton and an electron.
The new particle discovered by Chadwick was destined to play a totally unexpected role, not only in the history of atomic science but also in the fate of nations. It immediately outmoded a previous concept of the nucleus that pictured it as a cluster of protons approximately half of which were neutralized by electrons crowded into the nucleus. A nucleus is now thought of as containing just protons and neutrons.
The neutron was also greeted by nuclear workers as a practically perfect kind of bullet. Unlike charged alpha particles, uncharged neutrons can approach a charged nucleus completely unopposed. It is physically impossible for any kind of container to hold a swarm of free neutrons; they seep right through its walls.
Matter Is Energy; Energy Is Matter
So far, in the story about man’s curiosity concerning the fundamental nature and structure of matter, the development of ideas about structure has been emphasized. We will now take a brief look at a development which strongly influenced our ideas about the fundamental nature of matter.
In 1887 reports appeared on a famous study, often referred to as the Michelson-Morley experiment, which was aimed at determining the earth’s speed through absolute space. The entirely unexpected results of the experiment had a great impact on the concepts of space and time. We will here concern ourselves with just one outcome of the experiment.
In 1905, a young German-born physics student named Albert Einstein, who was working as a patent examiner in Switzerland, published three papers, each of which had a profound effect on a different field of physics.
One of the papers dealt with some peculiar speculations about space and time which began to interest him when he was studying the Michelson-Morley experiment. The contents of the paper are now referred to as the Special Theory of Relativity. This paper contains several predictions that seemed incredible to the average physicist of that day. These predictions have, however, long since been proved valid.

Albert Einstein in 1905.
Courtesy Lotte Jacobi, Hillsboro, New Hampshire
One of Einstein’s predictions had to do with the equivalence of matter and energy. Until 1905 matter had been considered as something that has mass or inertia; energy, on the other hand, had been regarded as the ability to do 15 work. It was believed that the two were as different from each other as, say, a square yard is different from an hour. Einstein’s theory, however, implies that matter and energy are merely two different manifestations of the same fundamental physical reality, and that each may be converted into the other according to the famous equation:
E = MC²
where
E = quantity of energy,
M = quantity of matter, and
C = speed of light in a vacuum.
Nuclei Contain Energy
One more piece of information must be fitted into the story of the atom before it becomes clear why some people began to realize during the 1920s that atomic nuclei contain vast stores of energy that might some day revolutionize civilization. This last item has to do with a nuclear phenomenon known as the packing fraction.
Since any nucleus consists of a certain number of protons and neutrons, it seems logical that the total weight of the nucleus could be determined by adding together the individual weights of the particles in it. When mass spectrographs of sufficiently high accuracy became available, however, it was found that in the case of nuclear weights, the whole was not equal to the sum of its parts! All nuclei (except hydrogen) weigh less than the sum of the weights of the particles in them.
For example, the atomic weight of a proton is 1.00812 and that of a neutron is 1.00893. (These are relative weights based on an internationally accepted scale.) It would seem then that a nucleus of helium containing two protons and two neutrons should have an atomic weight of 2 × 1.00812 plus 2 × 1.00893 or 4.0341. Actually the atomic weight of helium as measured by the mass spectrograph is only 4.0039. (See Figure 4.)
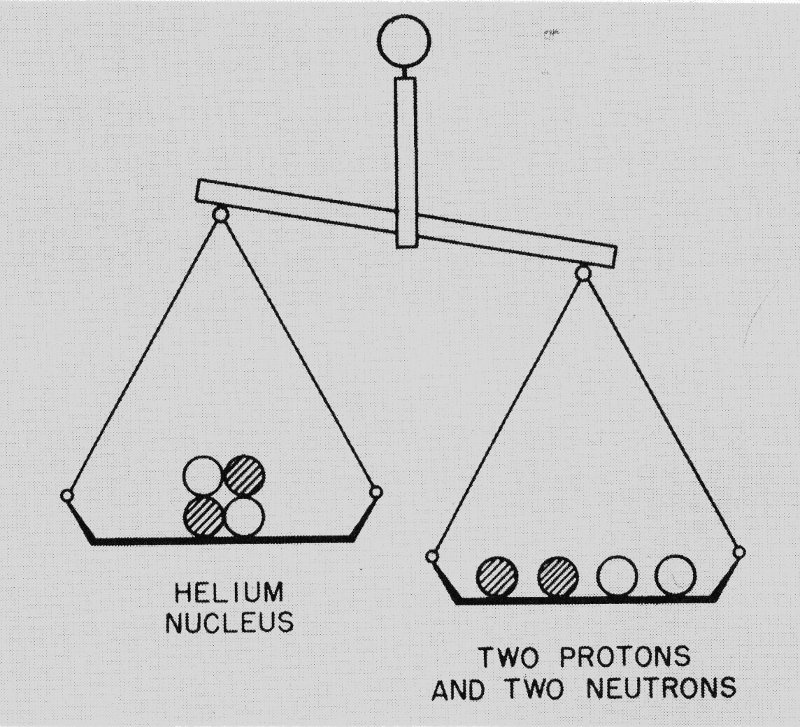
Figure 4 A case where the whole is not equal to the sum of its parts. Two protons and two neutrons are distinctly heavier than a helium nucleus, which also consists of two protons and two neutrons. Energy makes up the difference.
- HELIUM NUCLEUS
- TWO PROTONS AND TWO NEUTRONS
What happens to the missing atomic weight of 0.0302? Physicists now realize that, as postulated in Einstein’s formula, it must be converted into energy! The conversion occurs when the protons and neutrons are drawn together into a helium nucleus by the powerful nuclear forces between them.
When the missing atomic weight 0.0302 is multiplied by the square of the velocity of light according to Einstein’s theory, it is found to represent a tremendous amount of energy. Indeed, the energy released in forming a helium nucleus from two protons and two neutrons turns out to be seven million times that released when a carbon atom combines with an oxygen molecule to produce a molecule of carbon dioxide in the familiar process of combustion.
The general behavior of such losses in atomic weight for atoms throughout the periodic table had been determined as early as 1927, largely through the work of Aston, the English scientist who developed the first mass spectrograph. His results show that, in general, if two light nuclei combine to form a heavier one, the new nucleus does not weigh as much as the sum of the original ones. This behavior continues up to the level of the so-called “transition metals”—iron, 17 nickel, and cobalt—in the periodic table. But if two nuclei heavier than iron are coalesced into a single very heavy nucleus found near the end of the periodic table (such as uranium), the new nucleus weighs more than the sum of the two nuclei that formed it.
Thus, if a very heavy nucleus could be divided into parts, energy would be released, and the sum of the weights of the fragments would be less than that of the original nucleus.
In these two types of nuclear reactions, a small amount of matter would actually vanish! Einstein’s Special Theory of Relativity states that the vanished matter would reappear as an enormous quantity of energy.
During the late 1920s scientists began saying that a small amount of matter could supply enough energy to drive a large ship across the ocean. As we know, this prediction has since been borne out by the performance of nuclear submarines and surface vessels.

The NS Savannah was the first cargo-passenger ship to be driven by nuclear power.
Courtesy States Marine Lines

The Nautilus was the Navy’s first atomic-powered submarine.
Courtesy U. S. Navy
CHRONOLOGY
| 1800 | Dalton firmly establishes atomic theory of matter. |
| 1890-1900 | Thomson’s experiments with cathode rays prove the existence of electrons. Atoms are found to contain negative electrons and positive electric charge. Becquerel discovers unstable (radioactive) atoms. |
| 1905 | Einstein postulates the equivalence of mass and energy. |
| 1911 | Rutherford recognizes nucleus. |
| 1919 | Rutherford achieves transmutation of one stable chemical element (nitrogen) into another (oxygen). |
| 1920-1925 | Improved mass spectrographs show that changes in mass per nuclear particle accompanying transmutation account for energy released by nucleus. |
| 1932 | Chadwick identifies neutrons. |
| 1939 | Discovery of uranium fission by German scientists. |
| 1940 | Discovery of neptunium by Edwin M. McMillan and Philip H. Abelson and of plutonium by Glenn T. Seaborg and associates at the University of California. |
| 1942 | Achievement of first self-sustaining nuclear reaction, University of Chicago. |
| 1945 | First successful test of an atomic device, near Alamagordo, New Mexico, followed by the dropping of atomic bombs on Hiroshima and Nagasaki, Japan. |
| 1946 | U. S. Atomic Energy Commission established by Act of Congress. |
| First shipment of radioisotopes from Oak Ridge goes to hospital in St. Louis, Missouri. | |
| 19 | |
| 1951 | First significant amount of electricity (100 kilowatts) produced from atomic energy at testing station in Idaho. |
| 1952 | First detonation of a thermonuclear bomb, Eniwetok Atoll, Pacific Ocean. |
| 1953 | President Eisenhower announces U. S. Atoms-for-Peace program and proposes establishment of an international atomic energy agency. |
| 1954 | First nuclear-powered submarine, Nautilus, commissioned. |
| 1955 | First United Nations International Conference on Peaceful Uses of Atomic Energy held in Geneva, Switzerland. |
| 1957 | First commercial use of power from a civilian reactor takes place in California. |
| Shippingport Atomic Power Plant in Pennsylvania reaches full power of 60,000 kilowatts. | |
| International Atomic Energy Agency formally established. | |
| 1959 | First nuclear-powered merchant ship, the Savannah, launched at Camden, New Jersey. |
| Commissioning of first nuclear-powered Polaris missile-launching submarine George Washington. | |
| 1961 | A radioisotope-powered electric power generator placed in orbit, the first use of nuclear power in space. |
| 1962 | Nuclear power plant in the Antarctic becomes operational. |
| 1963 | President Kennedy ratified the Limited Test Ban Treaty for the United States on October 7. |
| 1964 | President Johnson signed law permitting private ownership of certain nuclear materials. |
Fission is Explained

Enrico Fermi 1901-1954
Courtesy Chemical and Engineering News
Physicists welcomed the neutron as a bullet that could strike any nucleus, unopposed by electric repulsion. During the middle 1930s, a number of investigators, chief among them the Italian physicist Enrico Fermi, exposed many different isotopes of the chemical elements to beams of neutrons to see what would happen.
What usually happened was that the bombarded nuclei would absorb neutrons, emit alpha, beta, or gamma rays, and change into different isotopes. The identification of the extremely small quantities of isotopes produced required the development of a fantastic new branch of chemistry known as radiochemistry, or, as one chemist put it, “phantom chemistry.”
In some cases the absorption of a neutron by a nucleus was followed by the emission of a negative electron (beta particle). This produced an atom whose nuclear positive charge had been increased by one unit and which therefore belonged at the next higher place on the periodic table. Fermi and others then considered the fascinating possibility of doing the same thing to uranium, the last-known element on the periodic table, to create previously unknown chemical elements. The results of bombarding uranium with neutrons turned out to be extremely complex, but it eventually became clear that “transuranic” elements (those heavier than uranium) could actually be made in this way.[2]
Some of the complex results of bombarding uranium with neutrons formed an intriguing puzzle that kept various investigators busy for several years. In 1939 the German chemists Otto Hahn and Fritz Strassmann and the physicists Lise Meitner and Otto Frisch were able to announce a solution. The absorption of a neutron by a certain uranium nucleus (later shown to be that of the relatively rare isotope uranium-235) can result in a splitting, or fission, of the nucleus into two parts with separate weights that place them somewhere near the middle of the periodic table.

Lise Meitner and Otto Hahn in their laboratory in the 1930s.
Courtesy Addison-Wesley Publishing Co.
The announcement of this discovery created quite a stir among physicists because a nuclear process of this nature must release a very large amount of energy.
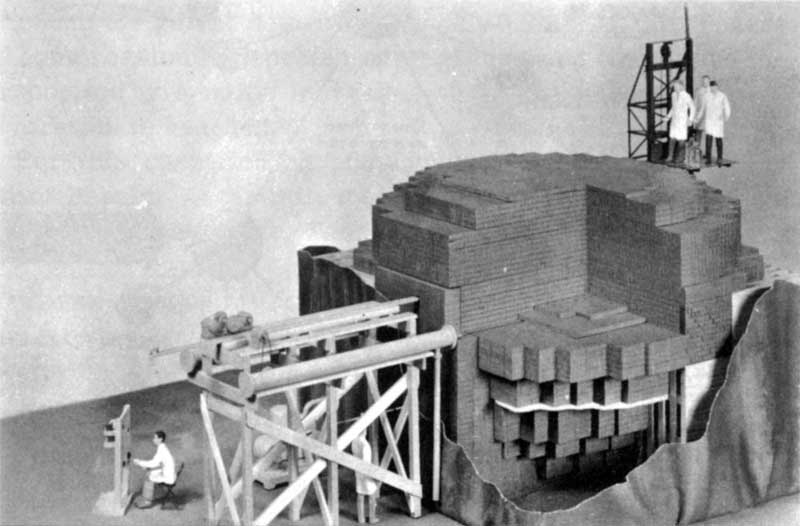
Scale model of the CP-1 (Chicago Pile No. 1) used by Enrico Fermi and his associates on December 2, 1942, to achieve the first self-sustaining nuclear reaction. Alternate layers of graphite, containing uranium metal and/or uranium oxide, were separated by layers of solid graphite blocks. Graphite was used to slow down neutrons to increase the likelihood of fissions.
The excitement among physicists became even greater when it was realized that this newly discovered process of 22 fission was accompanied by the release of several free neutrons from the splitting nucleus. Each new neutron could, if properly slowed down by a moderating material, cause another nucleus to split and release more energy and still more neutrons, and so on, as illustrated in Figure 5. (A moderator is necessary because fast, newly released neutrons are too readily absorbed by uranium-238 nuclei, which rarely split.) Apparently all that was needed to achieve this spectacular kind of a chain reaction was to assemble enough uranium in one place so that the released neutrons would have a good chance of finding another ²³⁵U nucleus before escaping from the pile. The amount of fissionable material required to sustain a chain reaction is termed the “critical mass.” A team of scientists led by Fermi achieved the first self-sustaining nuclear reaction on December 2, 1942, under the grandstand at the University of Chicago’s athletic field. This date is often referred to as the beginning of the Nuclear Age.
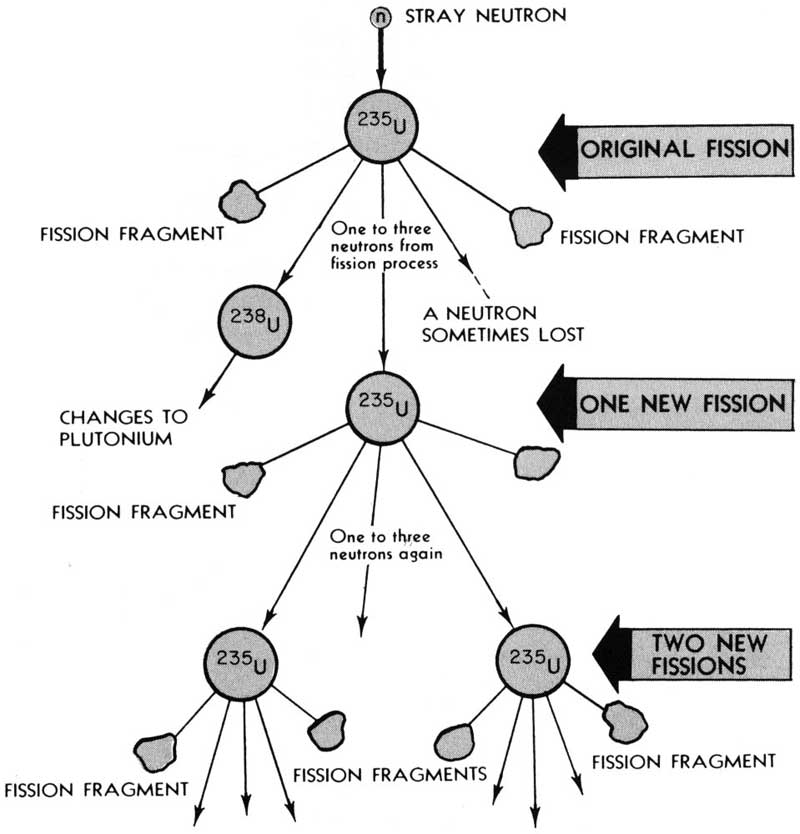
Figure 5 This diagram shows what happens in a chain reaction resulting from fission of uranium-235 atoms.
- STRAY NEUTRON
- ²³⁵U
- ORIGINAL FISSION
- FISSION FRAGMENTS
- One to three neutrons from fission process
- A NEUTRON SOMETIMES LOST
- ²³⁸U
- CHANGES TO PLUTONIUM
- ²³⁵U
- ONE NEW FISSION
- FISSION FRAGMENT
- One to three neutrons again
- ²³⁵U
- ²³⁵U
- TWO NEW FISSIONS
- FISSION FRAGMENTS
The Fission Bomb Is Exploded
The American scientists present on that historic December day were part of the tremendous super-secret scientific and industrial complex that bore the unrevealing title Manhattan District. The United States had been at war almost a year. An uncontrolled fission reaction gave promise of producing an explosion of untold proportions. This promise, coupled with the possibility that enemy scientists might be nearing such a goal, had launched a vast Allied effort.
The Manhattan Project, as it was commonly known, included a variety of “hush-hush” facilities. Each of these installations, in New York, Illinois, Tennessee, New Mexico, California, and Washington, had its own experts working night and day to solve the baffling problems surrounding development of a fission weapon.
Ordinary uranium as found in nature was not suitable for an atomic bomb because less than one percent of the atoms in it are fissionable isotope ²³⁵U.[3] It therefore became necessary to find some means for separating the rare ²³⁵U from the large quantity of ²³⁸U. Chemistry could not do it since the two isotopes are identical chemically.
Several methods of achieving large-scale separation were tried. The most successful and economical, known as “gaseous diffusion,” involves compressing normal uranium, in the form of uranium hexafluoride gas, against a porous barrier containing millions of holes, each smaller than two-millionths of an inch. Since the ²³⁵U molecules are slightly lighter than the ²³⁸U, they bounce against the barrier more frequently and have a greater chance of penetrating. Thus, although the gas at first contains only 0.7% ²³⁵U, the process of compression is repeated several thousand times, and the proportion gradually increases until the necessary concentration is reached.
For this operation an enormous plant containing a very large barrier area, miles of piping, and countless pumps was built at Oak Ridge, Tennessee.
At the same time that vast efforts were being made to produce a ²³⁵U bomb, another project of equal importance was being pursued to develop a different kind of fission bomb. Uncertainty as to whether it would be possible to separate usable amounts of ²³⁵U led to a decision to exploit a highly significant discovery about one of the transuranic elements.
By 1941 Glenn T. Seaborg, Edwin M. McMillan, Philip H. Abelson, and others at the Radiation Laboratory, Berkeley, California, had identified isotopes of two new transuranic elements developed when they bombarded ²³⁸U nuclei with neutrons. The new elements were named neptunium and plutonium after the planets Neptune and Pluto, which lie beyond Uranus in the solar system.[4] One isotope of plutonium, plutonium-239, which resulted from the absorption of a neutron by a ²³⁸U nucleus and the emission of two beta particles, was discovered to be as fissionable as ²³⁵U and hence theoretically just as feasible for a bomb. Since plutonium is chemically different from uranium, it offered the tremendous advantage that it could readily be concentrated by conventional chemical techniques.
The way to manufacture usable amounts of plutonium, an element that had never before been detected on earth, is to expose uranium to a very intense neutron bombardment. The best-known place to find a rich supply of neutrons was the heart of a self-sustaining chain-reacting pile of uranium. Accordingly, very large piles, or reactors, were rushed to completion near the Columbia River at Hanford, Washington, to make plutonium.

First atomic bomb explosion at Alamagordo, New Mexico, at 5:30 a.m. on July 16, 1945.
Courtesy U. S. Army
On July 16, 1945, a plutonium bomb, carefully assembled by another group of scientists at “Project Y,” Los Alamos, New Mexico, was successfully tested in the New 25 Mexico desert. The heat from that first man-made nuclear explosion completely vaporized a tall steel tower and melted several acres of surrounding surface sand. The flash of light was the brightest the earth had ever witnessed.
A ²³⁵U bomb was dropped on Hiroshima, Japan, on August 6, 1945. Three days later a plutonium bomb was dropped on Nagasaki, Japan. Hostilities ended on August 14, 1945.
Nuclear Energy Is Needed for the Future
The chief source of the enormous quantities of energy used daily by modern civilization is fossil fuels in the form of coal, petroleum, and natural gas. Concentrated sources of these fuels, though large, are far from inexhaustible, and it has been said that future historians may refer to the brief time when they were used as “the fossil-fuel incident.”

These lights of downtown Pittsburgh are symbolic of the generation of electricity by atomic power from Shippingport, Pennsylvania, the site of the world’s first full-scale atomic-electric generation station exclusively for civilian needs. Homes and factories of the greater Pittsburgh area are receiving the electricity produced at the plant and transmitted through the Duquesne Light Company system. The Shippingport plant is a joint project of Westinghouse Electric Corporation, U. S. Atomic Energy Commission, and the Duquesne Light Company.
Courtesy Westinghouse Electric Corporation
The next great source of energy will probably be nuclear reactors, in which controlled chain reactions release energy from the large store of fissionable materials in the world.[5]
The accomplishments of nuclear power in the propulsion of ships have already been noted. In addition, there is now going on in industrialized countries in different parts of the world a large-scale development of nuclear power plants for production of electricity. Nuclear electric power is approaching the point where it will be economically competitive with power from hydroelectric plants or those burning coal, oil, or gas as fuels. Improvements in nuclear power technology are rapidly being made, and it is now widely predicted that before the end of this century most new electric power plants will be nuclear.
Fusion Has Potential
One of the greatest puzzles to be solved by physicists arose from the work of geologists. When it became clear that coal and other fossil remains of living things date from many hundreds of millions of years ago, it was obvious that the earth’s sun had been shining at a quite steady rate for an extremely long time.
How does it manage to do it? What is its source of energy? Chemical energy supplied by combustion and gravitational potential energy supplied by contraction are thousands of times too small to have kept the sun going for such a long time.
The principle illustrated by Figure 4 suggests the most probable source of energy for the sun and all the other stars as well. It is known that the sun consists chiefly of hydrogen and that it has a temperature of about 40,000,000 degrees Fahrenheit near its center. Several kinds of nuclear reactions produced in atom smashers have demonstrated that hydrogen nuclei, if energized by being heated to a very high temperature, can actually combine, or fuse, to form helium nuclei.
The accompanying loss of weight per particle indicated by Figure 4 must result in the appearance of sufficient energy to balance Einstein’s famous equation. In fact, calculations by the German-born American physicist Hans A. Bethe and others show that, based on reasonable estimates 27 of the conditions within the sun, familiar nuclear reactions account for its energy. The calculations predict, furthermore, that the sun can continue to operate at its present level for many billions of years.
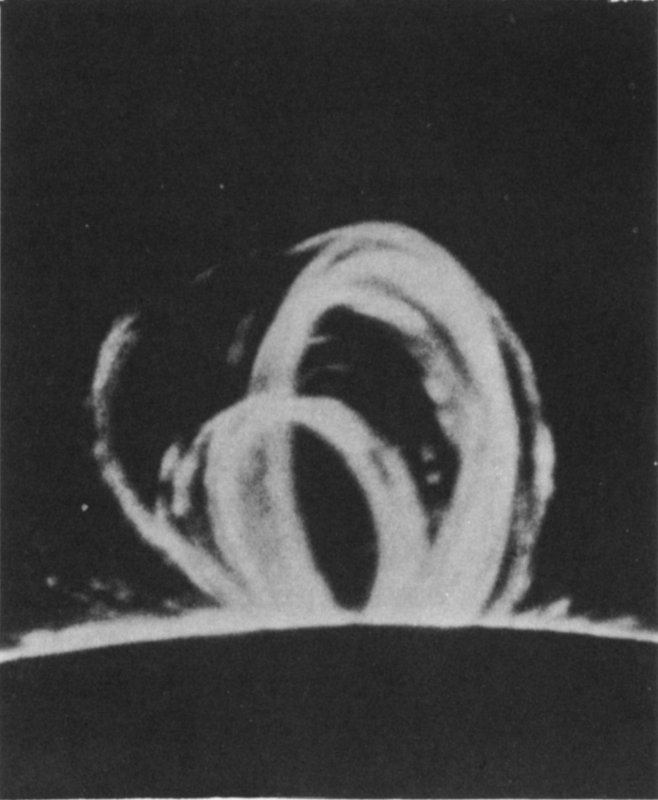
Large loop prominences on the sun, caused by a locally intense magnetic field. Project Sherwood, the U. S. program in controlled fusion, is devoted to research on fusion reactions similar to those from which the sun derives its energy.
Courtesy Sacramento Peak Observatory, AFCRL
Since fusion of light nuclei is produced by extremely high temperatures, fusion events are called thermonuclear reactions. The possibility of bringing about thermonuclear reactions on earth to serve as a source of energy has naturally attracted much attention.
In spite of the fact that fusion of ordinary hydrogen atoms (each of which has one proton as its nucleus) supports the activity of the sun, this particular reaction seems to occur much too slowly to be usable on earth. Other isotopes of hydrogen, called deuterium and tritium, however, which contain one and two neutrons in their nuclei, respectively, fuse much more rapidly and seem to be potential earthly sources of controlled thermonuclear energy.

An early phase of a nuclear detonation at Eniwetok Atoll during the 1951 tests.
Courtesy Joint Task Force Three
The first large-scale application of thermonuclear energy was the so-called hydrogen bomb, or “H-bomb.” For a brief time an exploding fission bomb develops a temperature 28 of hundreds of millions of degrees Fahrenheit, hot enough to cause some light nuclei to fuse. In the hydrogen bomb, light nuclei of deuterium and/or tritium are exposed to this temperature during such a fission explosion. The resulting fusion of these nuclei causes the explosion to be hundreds of times more powerful than that of the fission device alone. In 1952 the Atomic Energy Commission test-fired such a thermonuclear device at Eniwetok Atoll in the Pacific Ocean. The energy released by the highly efficient device produced an explosion that completely destroyed the coral islet where it was detonated.
At such extreme temperatures all atoms are stripped of electrons; the resulting mixture of nuclei and free electrons is called a plasma. Several laboratories are now working on the problems connected with creating and containing plasma. Ordinary solid containers cannot be used. On contact with plasma they would instantly vaporize and would cool the plasma below the temperature necessary for fusion to occur. Fortunately, however, the particles that make up a plasma, being charged electrically, respond to forces in a magnetic field. A strong magnetic field of proper shape exerts a large confining pressure on a body of plasma in a high-vacuum chamber. Thus plasma can be contained in a small volume well removed from the walls of the chamber by surrounding the chamber with suitably designed large magnets or solenoids to create a “magnetic bottle.” In addition, a sudden increase in the intensity of the field can compress the plasma; this compression raises the temperature of the plasma to near that required for fusion.
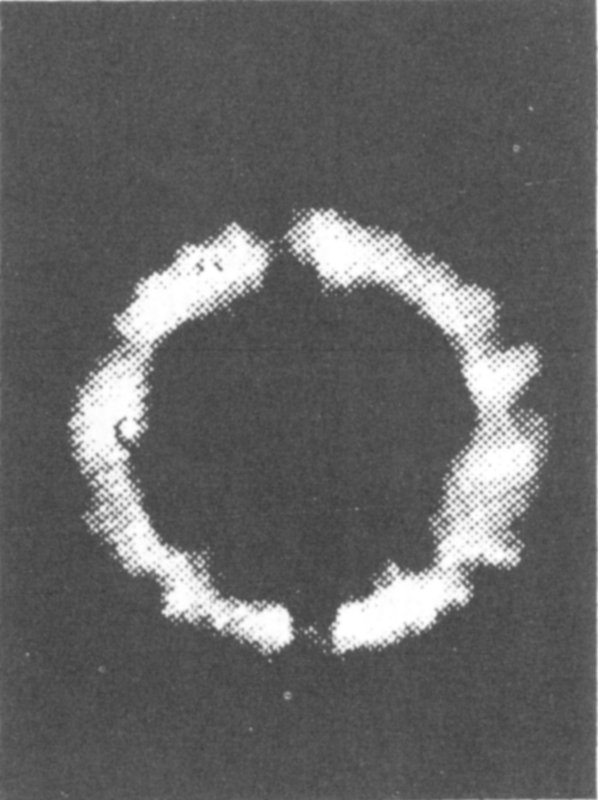
This plasma is being pushed outward by an internal magnetic field as instabilities grow on its internal surface. The photo was taken by means of fast-shutter photography permitting photo sequences at intervals of 3 to 5 millionths of a second.
Courtesy General Atomic Division, General Dynamics Corporation
Fusion of light nuclei would be a much “cleaner” source of energy for peaceful purposes than fission of heavy ones, because the “ashes” of fission reactions are radioactive while those of fusion (helium atoms) are not. Great technical difficulties must be overcome, however, before a controlled thermonuclear reaction is possible. Fusionable material must be heated to a temperature of over 100 million degrees Fahrenheit and must be contained long enough for an appreciable amount of fusion to occur.
The greatest problem encountered to date is the extreme instability of the plasma and the corresponding difficulty of maintaining it at the proper temperature longer than a few millionths of a second. Many physicists now think that the successful exploitation of thermonuclear energy will not occur for many years. When and if it is achieved, however, the deuterium present in the oceans of the earth will represent an almost inexhaustible source of energy.
Isotopes Have Many Uses
The ability to produce and control nuclear reactions is affecting, and will doubtless continue to affect, human life in two outstanding ways. One way is by making tremendous amounts of energy available, either as explosions or as energy released from controlled reactions for peacetime use. The other way is by producing a vast variety of radioactive isotopes, first in the particle accelerators (“atom smashers”) mentioned earlier, and now in large quantities in nuclear reactors.
The presence of a radioactive isotope can be detected by instruments like the familiar Geiger counter; for this reason isotopes make wonderful tracers. These telltale atoms, which, in effect, continually cry “Here I am,” can trace the course of a chemical element through any kind of chemical reaction. Chemists are taking advantage of this new way of tagging atoms to study reaction patterns that, heretofore, have been obscure.
As a consequence, a scientist’s ability to synthesize scarce chemicals is being increased. The exact role of numerous essential trace elements in the growth and metabolism of living things, including people, is being studied by the use of tagged atoms.
Radioisotopes at Work
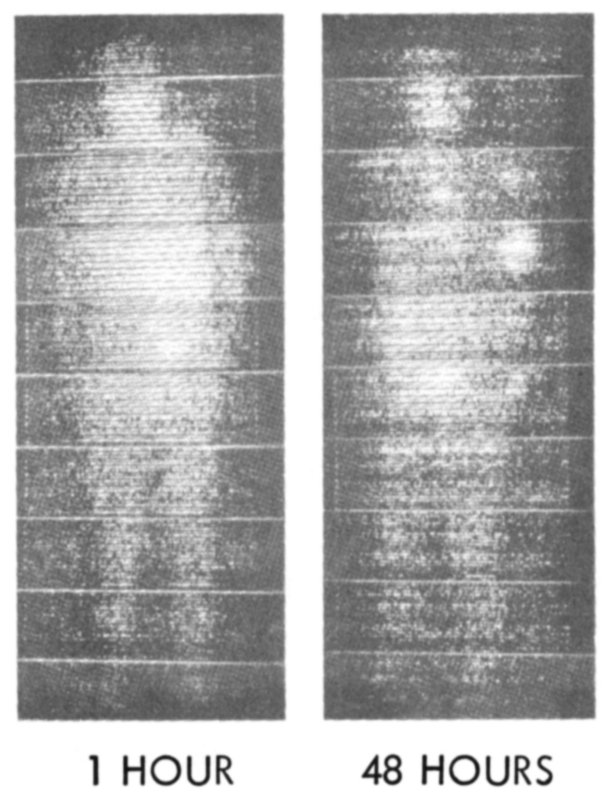
IN MEDICINE: Iodine-131 reveals spread of thyroid cancer in patient’s body.
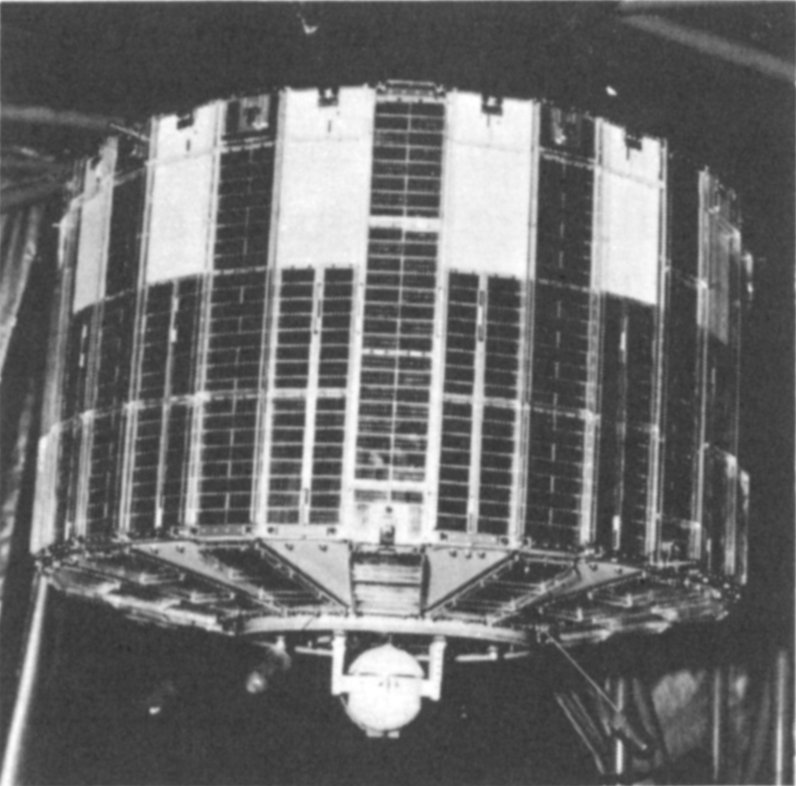
IN SPACE: Plutonium-238 is the fuel for the atomic generator powering this TRANSIT satellite.
Courtesy The Martin Company
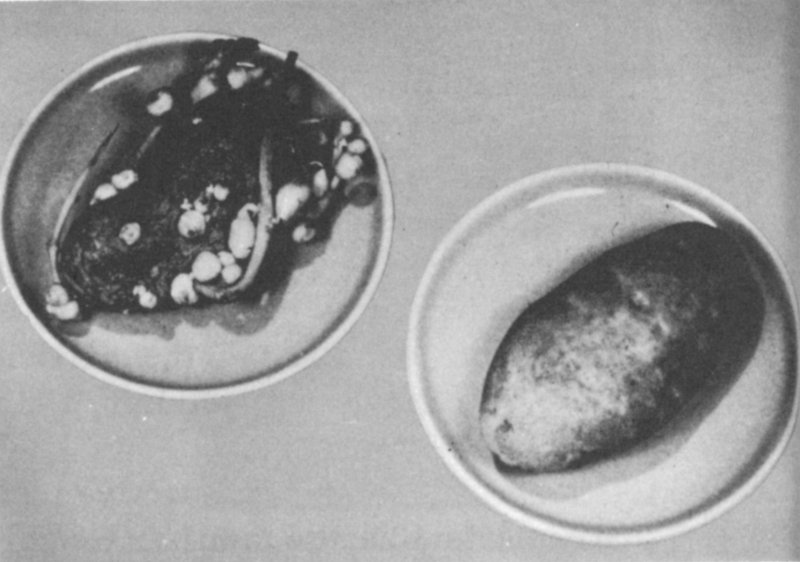
IN FOOD PRESERVATION: Potatoes stored for 18 months at 47°F. Potato at right had been irradiated, that on left had not.
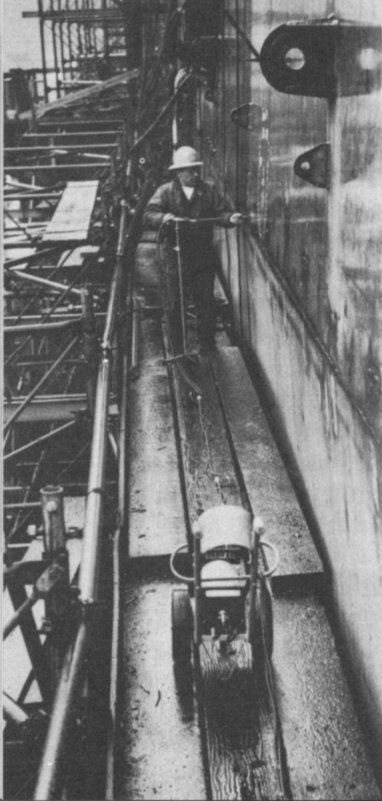
IN INDUSTRY: Radioactive iridium was used to inspect the hull of the carrier Independence.
Courtesy Technical Operations, Inc.
As sources of radiation, radioactive isotopes are frequently replacing more expensive and less convenient sources such as radium and X-ray machines. The medical treatment of diseased tissue has been greatly expedited by the new sources. In industry many applications of radiation sources have been made. They are used, for example, in thickness gauging and in making radiographs to check the quality of large castings. The sterilization and preservation of food is another promising use for inexpensive radioactive sources.
As a controllable means for inducing genetic mutations, radioactive isotopes are speeding up the process of selecting and developing superior agricultural products. Practically every agricultural research center in the world has one or more projects under way which involve the use of isotopes.
Small devices have also been constructed which produce electricity from heat generated by decay of radioisotopes. Such devices have been used to power instruments in a remotely located unmanned weather station, a navigational buoy, a lighthouse, an underwater navigational beacon, and space satellites. Many additional uses are foreseen for these isotopic power generators.
The Atomic Energy Commission
Following the end of World War II a vigorous controversy developed as to whether atomic energy development in the United States should continue under military control or be transferred to civilian control. The proponents of civilian control won out, and a civilian Atomic Energy Commission was established by the Atomic Energy Act of 1946. Under this Act, which was amended in 1954, the AEC manufactures nuclear weapons for the armed services; produces fissionable materials for both military and civilian purposes; fosters research and development in the basic sciences underlying atomic energy and in applications such as power 32 production and uses of radioisotopes; regulates the activities of private organizations using atomic energy; and distributes information about atomic energy. (This booklet is a small example; most of the information distributed is much more detailed and technical.)

President Truman signs the bill creating the U. S. Atomic Energy Commission on August 1, 1946. Behind the President, left to right: Senators Tom Connally, Eugene D. Millikin, Edwin C. Johnson, Thomas C. Hart, Brien McMahon, Warren R. Austin, and Richard B. Russell.
Courtesy United Press International
Almost all of the AEC’s materials production and research and development activities are carried out under contract by other organizations. American industry, universities, and research organizations also are engaged in widespread atomic energy activities of their own, subject only to such government regulations as are needed to protect national security and public health and safety. For example, the largest atomic electric power plants now in operation in this country are privately owned, as are numerous small atomic reactors used for research. At the end of 1962 some 7000 firms, institutions or individuals in the United States held federal or state licenses giving them permission to use radioisotopes. The number of persons employed in atomic energy work in the United States is estimated to be about 140,000, of which only 8000 work for the Federal Government.
Toward an International Atom
In December 1953, President Eisenhower, in a memorable address to the General Assembly of the United Nations, proposed the establishment under the aegis of the United Nations of an International Atomic Energy Agency “to serve the peaceful pursuits of mankind.” This proposal captured the imagination of people everywhere, and negotiations soon began as to the purpose, structure, scope, and program of such an organization. In October 1956 an 81-nation United Nations conference unanimously adopted a statute for the agency, which came into existence a year later with headquarters in Vienna, Austria. By the end of 1962 the IAEA had 78 member countries. Its most important work has been assisting some of the less developed nations of the world to begin programs for peaceful use of atomic energy.

On December 8, 1953, President Dwight D. Eisenhower proposed before the United Nations General Assembly that an International Atomic Energy Agency be established through which all nations could share knowledge and materials to develop the peaceful uses of atomic energy for the benefit of all mankind. Seated on the presidential platform are, left to right, Mr. Dag Hammarskjöld, Secretary-General of the U. N., Madame Vijaya Lakshmi Pandit of India, President of the General Assembly, and Mr. Andrew Cordier, Executive Assistant to the Secretary-General.
Courtesy United Nations

This 150,000-kilowatt, dual-cycle, boiling-water reactor, located 35 miles north of Naples, Italy, on the Garigliano River, was built by General Electric under the United States-Euratom Joint Program. It achieved criticality on June 5, 1963.
Even before the international agency became an accomplished fact, the United States sought on its own to implement the spirit of President Eisenhower’s proposal. It initiated in 1955 an Atoms-for-Peace Program under which the United States has made bilateral agreements with some 40 nations for the sharing of information on peaceful uses of atomic energy and under which the United States has helped other nations to acquire nuclear reactors and materials for peaceful use.
Mention should also be made of the International Conferences on Peaceful Uses of Atomic Energy which the United Nations held in Geneva, Switzerland, in 1955, 1958, and 1964. The 1955 conference was particularly noteworthy in that it marked the first time that scientists had met on a worldwide basis to discuss atomic energy. At and following this meeting much information previously kept secret was made public.
Suggested References
Books
Atomic Energy, Irene D. Jaworski and Alexander Joseph, Harcourt, Brace and World, Inc., New York 10017, 1961, 218 pp., $4.95.
Atompower, Joseph M. Dukert, Coward-McCann, Inc., New York 10016, 1962, 127 pp., $3.50.
Atoms Today and Tomorrow (revised edition), Margaret O. Hyde, McGraw-Hill Book Company, New York 10036, 1966, 160 pp., $3.25.
Basic Laws of Matter (revised edition), Harrie S. W. Massey and Arthur R. Quinton, Herald Books, Bronxville, New York 10710, 1965, 178 pp., $3.75.
Building Blocks of the Universe (revised edition), Isaac Asimov, Abelard-Schuman, Ltd., New York 10019, 1961, 380 pp., $3.50 (hardback); $2.70 (paperback) from E. M. Hale and Company, Eau Claire, Wisconsin 54701.
Elements of the Universe, Glenn T. Seaborg and Evans G. Valens, E. P. Dutton and Company, Inc., New York 10003, 1958, 253 pp., $4.95 (hardback); $2.15 (paperback).
Inside the Atom (revised edition), Isaac Asimov, Abelard-Schuman, Ltd., New York 10019, 1966, 197 pp., $4.00.
Introducing the Atom, Roslyn Leeds, Harper and Row, Publishers, New York 10016, 1967, 224 pp., $3.95.
Peacetime Uses of Atomic Energy (revised edition), Martin Mann, The Viking Press, New York 10022, 1961, 191 pp., $5.00 (hardback); $1.65 (paperback).
The Useful Atom, William R. Anderson and Vernon Pizer, The World Publishing Company, Cleveland, Ohio 44102, 1966, 185 pp., $5.75.
Secret of the Mysterious Rays: The Discovery of Nuclear Energy, Vivian Grey, Basic Books, Inc., Publishers, New York 10016, 1966, 120 pp., $3.95.
The Heart of the Atom: The Structure of the Atomic Nucleus, Bernard L. Cohen, Doubleday and Company, Inc., New York 10017, 1967, 120 pp., $3.95 (hardback); $1.25 (paperback).
The Questioners: Physicists and the Quantum Theory, Barbara L. Cline, Thomas Y. Crowell Company, New York 10003, 1965, 274 pp., $5.00.
The Atom and Its Nucleus, George Gamow, Prentice-Hall, Inc., Englewood Cliffs, New Jersey 07632, 1961, 153 pp., $1.95.
The Atomic Energy Deskbook, John F. Hogerton, Reinhold Publishing Corporation, New York 10022, 1963, 673 pp., $11.00.
Atomic Energy Encyclopedia in the Life Sciences, Charles W. Shilling (Ed.), W. B. Saunders Company, Philadelphia, Pennsylvania 19105, 1964, 474 pp., $10.50.
Atoms for Peace (revised edition), David O. Woodbury, Dodd, Mead and Company, New York 10016, 1965, 275 pp., $4.50.
Manhattan Project, Stephane Groueff, Little, Brown and Company, Boston, Massachusetts 02106, 1967, 372 pp., $6.95.
The New World, 1939/1946, Volume 1—History of the United States Atomic Energy Commission, Richard G. Hewlett and Oscar E. Anderson, Jr., The Pennsylvania State University Press, University Park, Pennsylvania 16802, 1962, 766 pp., $5.50.
Sourcebook on Atomic Energy (third edition), Samuel Glasstone, D. Van Nostrand Company, Inc., Princeton, New Jersey 08540, 1967, 883 pp., $9.25.
The World of the Atom, 2 volumes, Henry A. Boorse and Lloyd Matz (Eds.), Basic Books, Inc., Publishers, New York 10016, 1966, 1873 pp., $35.00.
Motion Pictures
Available for loan without charge from the AEC Headquarters Film Library, Division of Public Information, U. S. Atomic Energy Commission, Washington, D. C., and from other AEC film libraries.
Each of the following motion pictures explains atomic structure, fission, and the chain reaction. Additional contents are listed below with the film.
A Is for Atom, 15 minutes, sound, color, 1964. Produced by the General Electric Company. This film discusses natural and artificially produced elements, stable and unstable atoms, principles and applications of nuclear reactors, and the benefits of atomic radiation to biology, medicine, industry, and agriculture. (Level: elementary through high school.)
Atomic Energy, 10 minutes, sound, black and white, 1950. Produced by Encyclopedia Britannica Films, Inc. The film explains nuclear synthesis and shows how, through photosynthesis, the sun’s energy is stored on earth and released through combustion. (Level: intermediate through high school.)
Controlling Atomic Energy, 13½ minutes, sound, color, 1961. Produced by United World Films, Inc. This film gives a summary explanation of the following: radioactive atoms, radioactivity measurement, nuclear reactors, and the production and application of radioisotopes in biology, medicine, industry, agriculture, and research. (Level: 5th through 8th grades.)
Introducing Atoms and Nuclear Energy, 11 minutes, sound, color, 1963. Produced by Coronet Instructional Films. This film discusses nuclear fusion in the sun and, very briefly, the uses of nuclear energy. (Level: 4th through 9th grades.)
Atomic Physics, 90 minutes, sound, black and white, 1948. Produced by the J. Arthur Rank Organisation, Inc. This film discusses in detail the history and development of atomic energy with emphasis on nuclear physics. Dalton’s basic atomic theory, Faraday’s early electrolysis experiments, and Mendeleev’s 37 periodic table, the investigation of cathode rays, discovery of the electron, how the nature of positive rays was established, and the discovery of X rays are among the historical highlights. Explanation is presented of the work of the Joliot-Curie’s and Chadwick in the discovery of the neutron, and the splitting of the lithium atom by Cockcroft and Walton. Einstein tells how their work illustrates his theory of equivalence of mass and energy. (Level: high school.)
Unlocking the Atom, 20 minutes, sound, black and white, 1950. Produced by United World Films, Inc. This film explains the properties of alpha, beta, and gamma rays, cyclotrons, and the contributions of various scientists. (Level: junior and senior high school.)
This “Understanding the Atom” series of semi-technical lecture films is designed for inclusion in a high school senior-level chemistry or physics course, or it could be used as an introductional unit in nuclear science at the college level. The films all have sound and are in black and white.
Alpha, Beta, and Gamma, 44 minutes, 1962.
Radiation and Matter, 44 minutes, 1962.
Radiation Detection by Ionization, 30 minutes, 1962.
Radiation Detection by Scintillation, 30 minutes, 1963.
Properties of Radiation, 30 minutes, 1962.
Nuclear Reactions, 29½ minutes, 1963.
Radiological Safety, 30 minutes, 1963.

